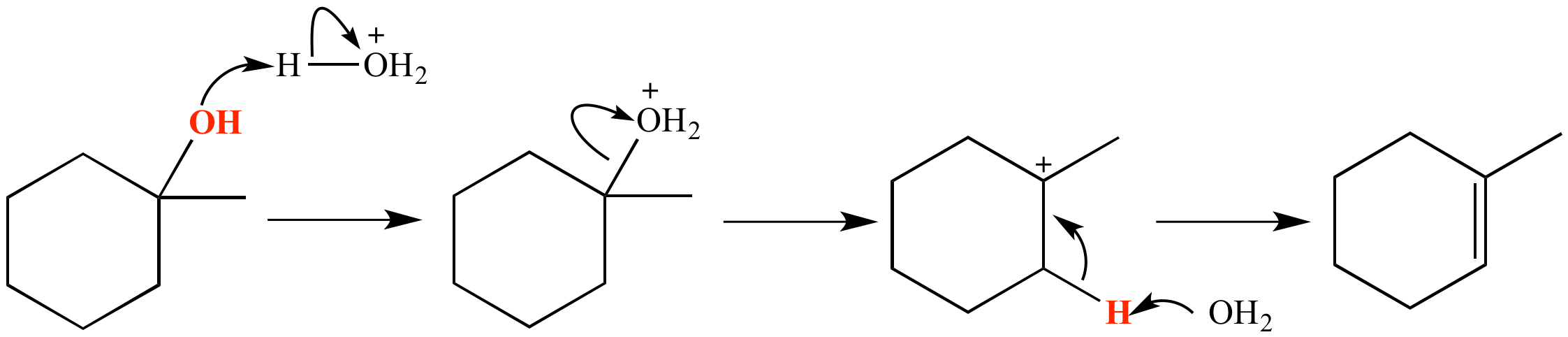
Reaction of 1-methyl-1-cyclohexanol with aqueous sulfuric acid results in dehydration and formation of an alkene product. The atoms which constitute the molecule of water that is removed are shown in red. The reaction follows the E1 mechanism, as shown in this example.
| CoCl2. 6 H2O | Anhydrous CoCl2 |
 |
 |
|
| Sucrose
+ H2SO4 |
Carbon
+ water
vapor (steam) |