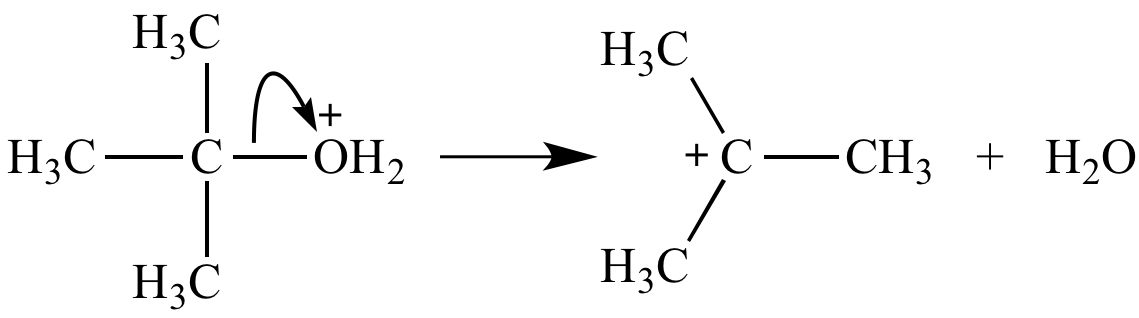
Oxonium ion fate #1: Ionization to form a carbocation. Water and alcohols are moderate leaving groups. Ease of this ionization depends strongly on the stability of the carbocation product.
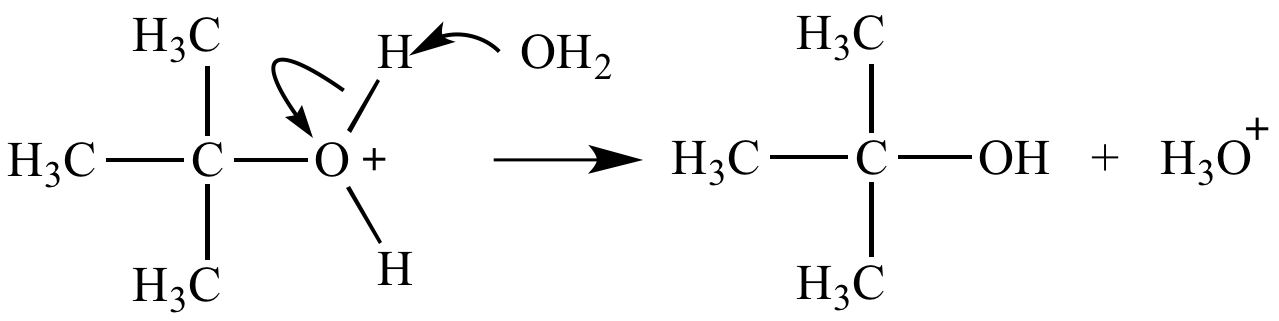
Oxonium ion fate #2: Deprotonation. An oxonium ion O-H bond is strongly acidic (R2OH+ pKa ~ -2).
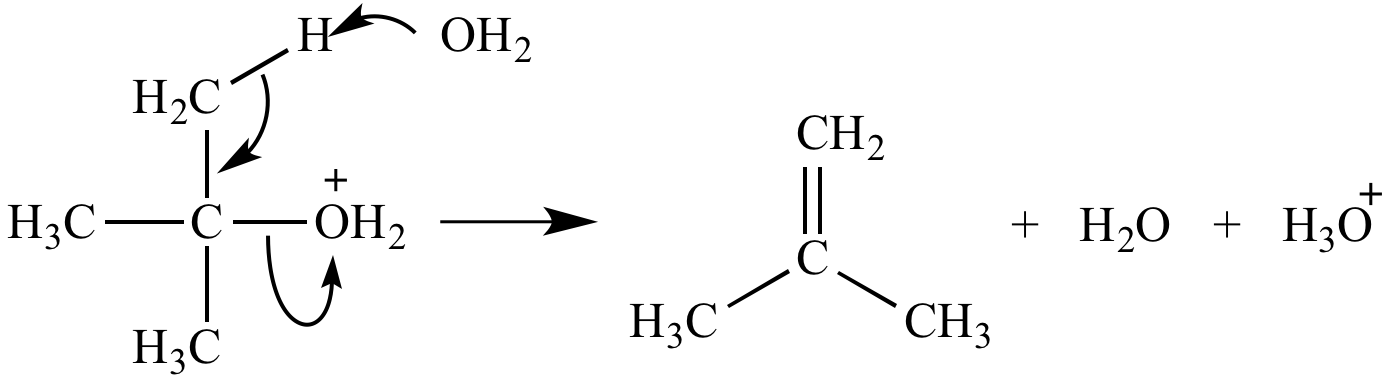
Oxonium ion fate #3: Elimination to form a pi bond. Water and alcohols are moderate leaving groups.
Oxonium ion fate #4a: Nucleophilic attack (at sp3 carbon). When the oxonium ion has an sp3 carbon with low steric hindrance, it can undergo an SN2 nucleophilic substitution.
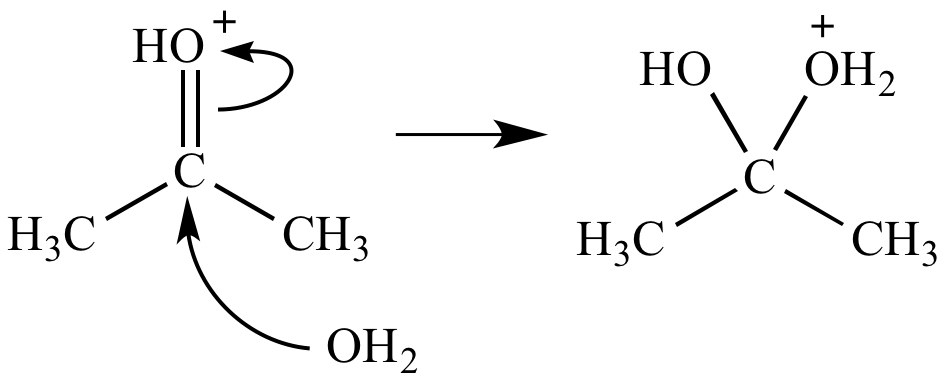
Oxonium ion fate #4b: Nucleophilic attack (at a carbonyl carbon). When the oxonium ion is a protonated carbonyl group, the carbonyl can accept a nucleophile at the carbon atom, producing a tetrahedral adduct. This is one of the three common carbonyl fates.