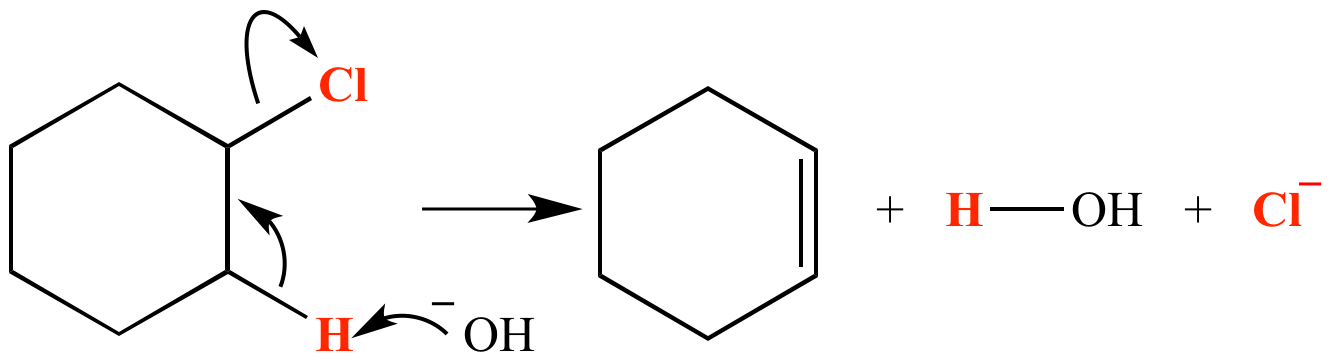
When reacted with strong base such as hydroxide ion, cyclohexyl chloride suffers dehydrohalogenation by a concerted E2 reaction mechanism (shown here). The atoms which comprise the molecule of HCl lost are shown in red. This reaction is the conceptual reverse of electrophilic addition of HCl to cyclohexene.
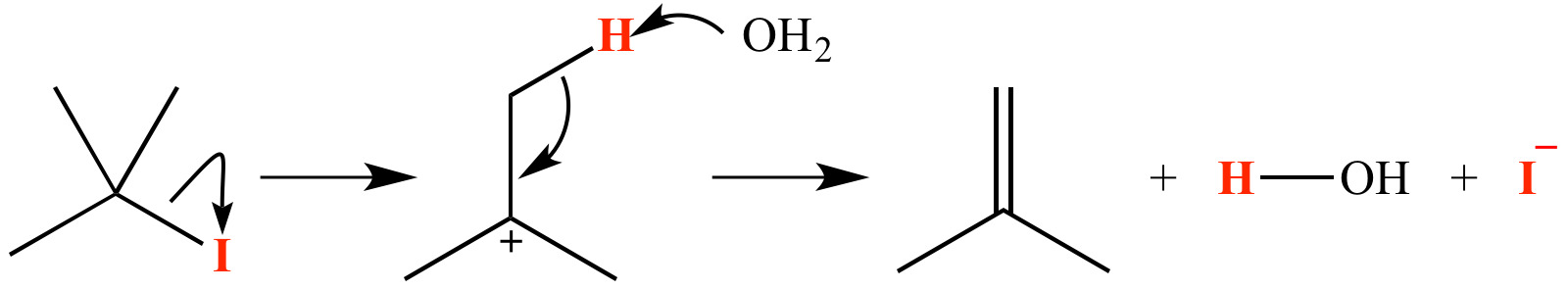
A tertiary alkyl halide such as 2-iodo-2-methylpropane can undergo dehydrohalogenation by the E1 reaction mechanism (shown here). The atoms which comprise the molecule of HI lost are shown in red.
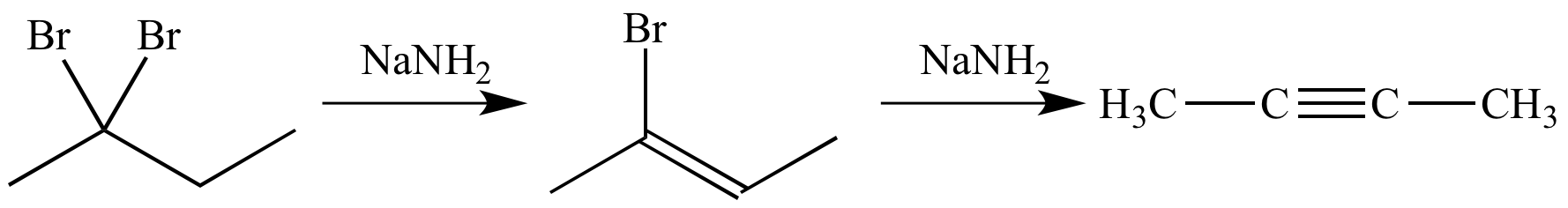
When reacted with a strong base such as sodium amide (NaNH2), a geminal dibromide can undergo a double dehydrohalogenation reaction to give an alkyne. The reaction involves a pair of consecutive E2 elimination steps.