
Carbonyl fate #1: Nucleophilic addition at the carbonyl carbon. This can occur because the carbonyl carbon has a δ+ charge, making the carbonyl group electrophilic. This nucleophilic addition produces a tetrahedral adduct. In the example shown here, acetone (the electrophile) reacts with hydroxide ion (the nucleophile), resulting in an oxyanionic tetrahedral intermediate.
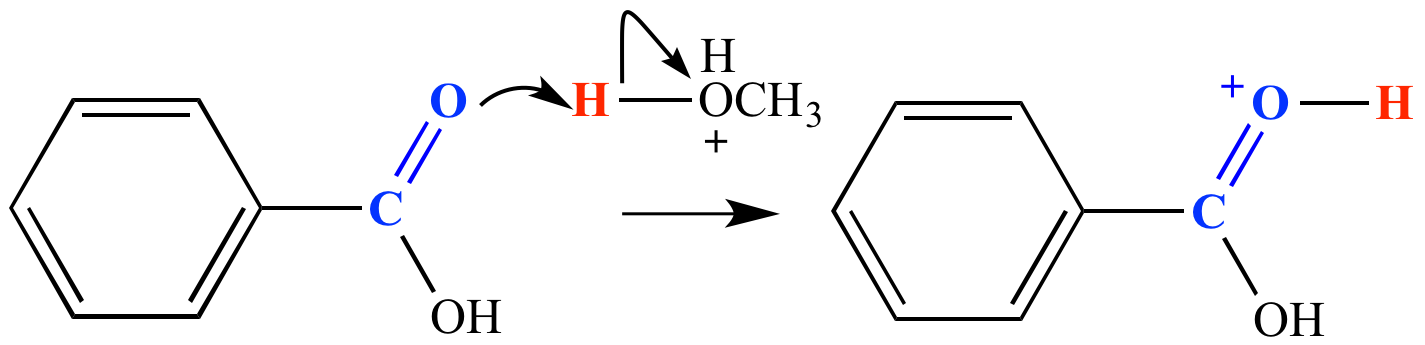
Carbonyl fate #2: Accept an electrophile (usually a proton) at the carbonyl group oxygen or the carbon-oxygen pi bond. (Protonation at either site leads to the same mechanism step product.) Carbonyl protonation requires a strong acid such as H3O+ (pKa -1.8). The protonation produces an oxonium ion. Other Lewis acids may bond with the carbonyl oxygen in some cases. In the example shown here, benzoic acid is protonated by CH3OH2+ (pKa -2). This is a step in the mechanism for the Fischer esterification reaction.
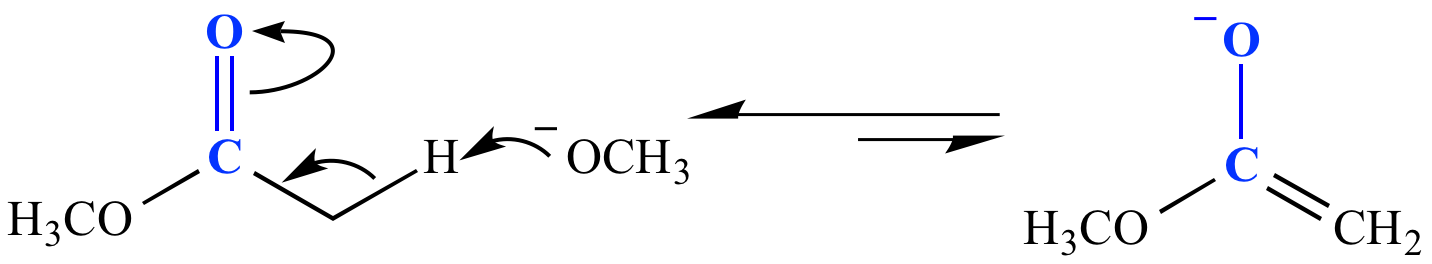
Carbonyl fate #3: Form an enolate. A base deprotonates a carbon adjacent to the carbonyl. The strength of base required to form the enolate, as well as how much enolate is formed, is controlled by the pKa of the proton to be deprotonated. In the example shown here, an ester (pKa 25) is deprotonated by methoxide ion (a strong base; CH3OH pKa 15.5). Only a tiny amount of enolate is formed (Keq ~10-10). This is the first step in the mechanism for a Claisen condensation reaction.