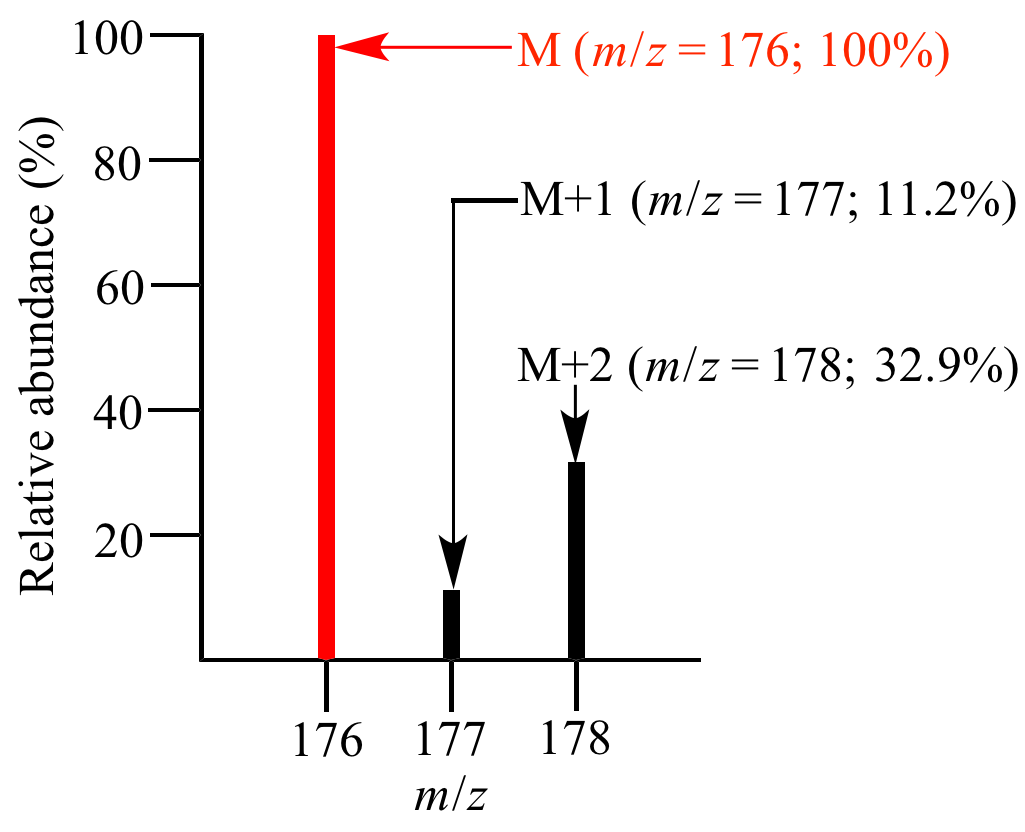
Molecular ion region of the mass spectrum of 1-chlorodecane (C10H21Cl).
The relative abundance of M is due to 12C101H2135Cl.
The relative abundance of M+1 is due to 12C913C1H2135Cl + 12C101H202H35Cl.
The relative abundance of M+2 is due to 12C914C1H2135Cl + 12C813C21H2135Cl + 12C913C1H202H35Cl + 12C101H192H235Cl + 12C101H193H35Cl + 12C101H2137Cl.