 |
 |
 |
 |
||||
| Molecule: |
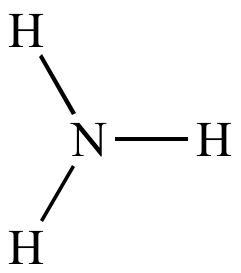
Ammonia |
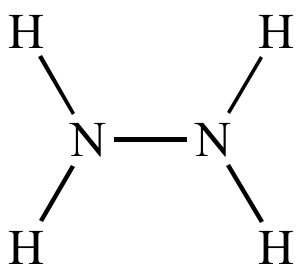
Hydrazine |
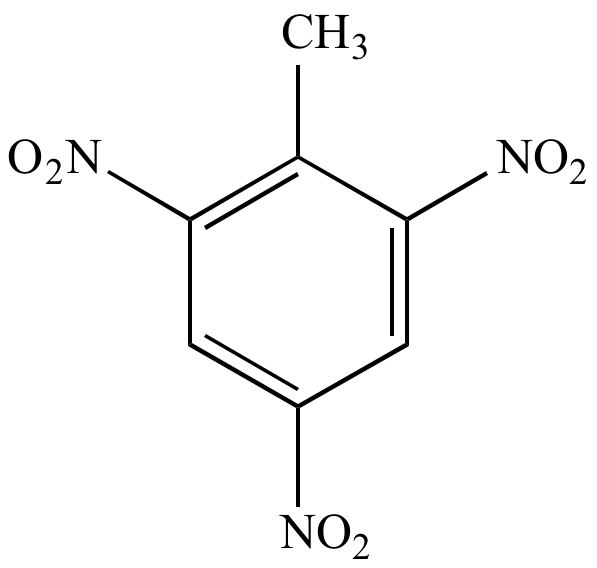
TNT |
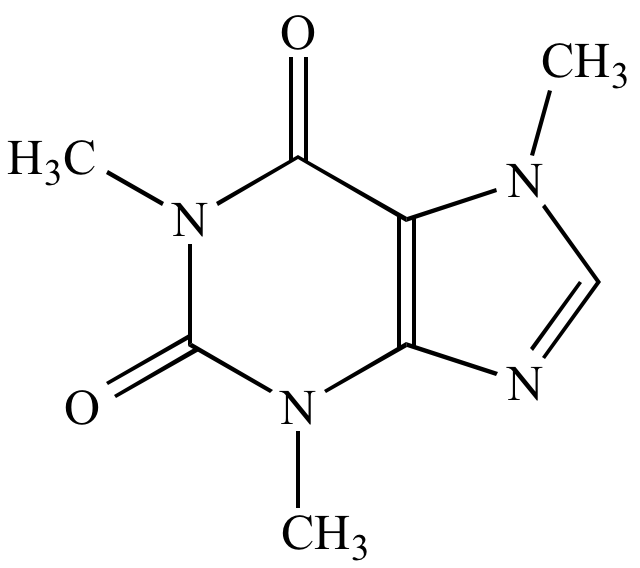
Caffeine |
|||
| Formula: |
NH3 |
N2H4 |
C7H5N3O6 |
C8H10N4O2 |
|||
| m/z (M): |
17 |
|
32 |
|
227 |
|
194 |
| One nitrogen m/z (M) is odd |
Two nitrogens m/z (M) is even |
Three nitrogens m/z (M) is odd |
Four nitrogens m/z (M) is even |