Chemical ionization
(CI; CI-MS):
In mass
spectrometry, a method for analyte
ionization
in which another molecule
(called the reagent gas) is ionized
by electron
impact, and then these reagent gas molecular
ions are impacted on the analyte
causing the analyte
to be ionized.
The reagent gas often methane
or ammonia.
Chemical ionization
imparts less kinetic energy to the analyte,
so compared to electron
impact
ionization, the relative amount of fragmentation
is reduced.
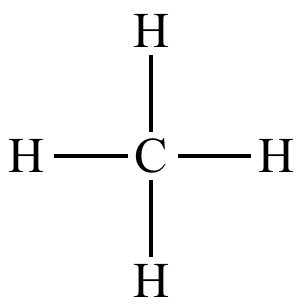 |
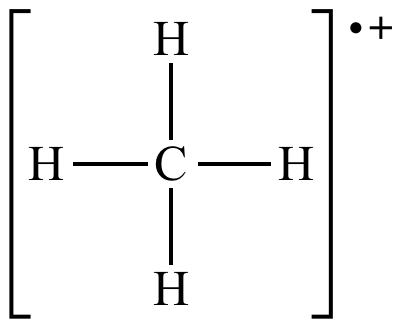 |
||||
| Methane |
Methane molecular ion |
Analyte molecular ion |