Hydrogen bonding
(H-bond):
A noncovalent
(van
der
Waals)
attractive force caused by electrostatic
attraction of a hydrogen atom with a lone
pair of another atom. The hydrogen
bond
donor must have a sufficiently large δ+
charge caused by bonding
to a highly electronegative
element (O, N, or F; or in uncommon cases by strong electron-withdrawing
inductive
effects). The hydrogen
bond
acceptor must have a lone
pair, and sufficiently high electron
density (the accepting atom must have a negative formal
charge, or if neutral must be oxygen or nitrogen).
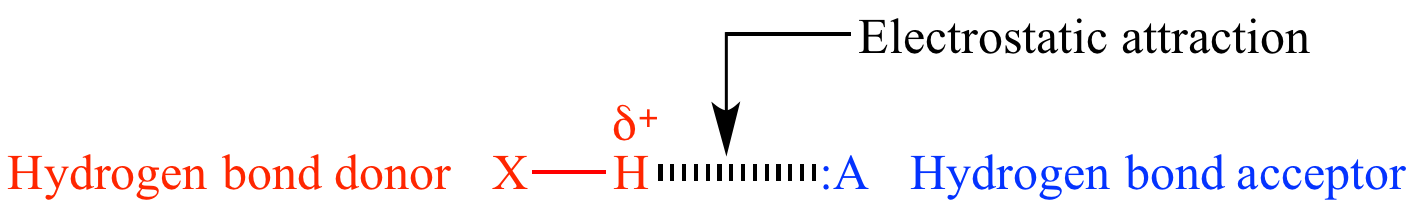 |
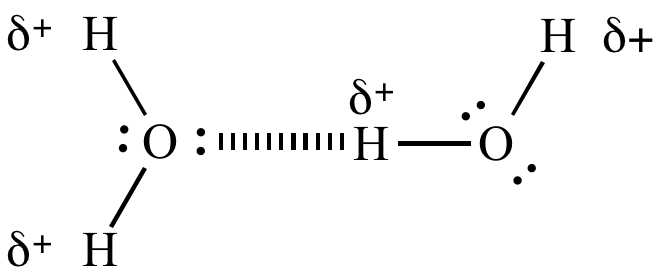 |
|
| General hydrogen bond structure. | |
Hydrogen bonding in water. |
 |
 |
|
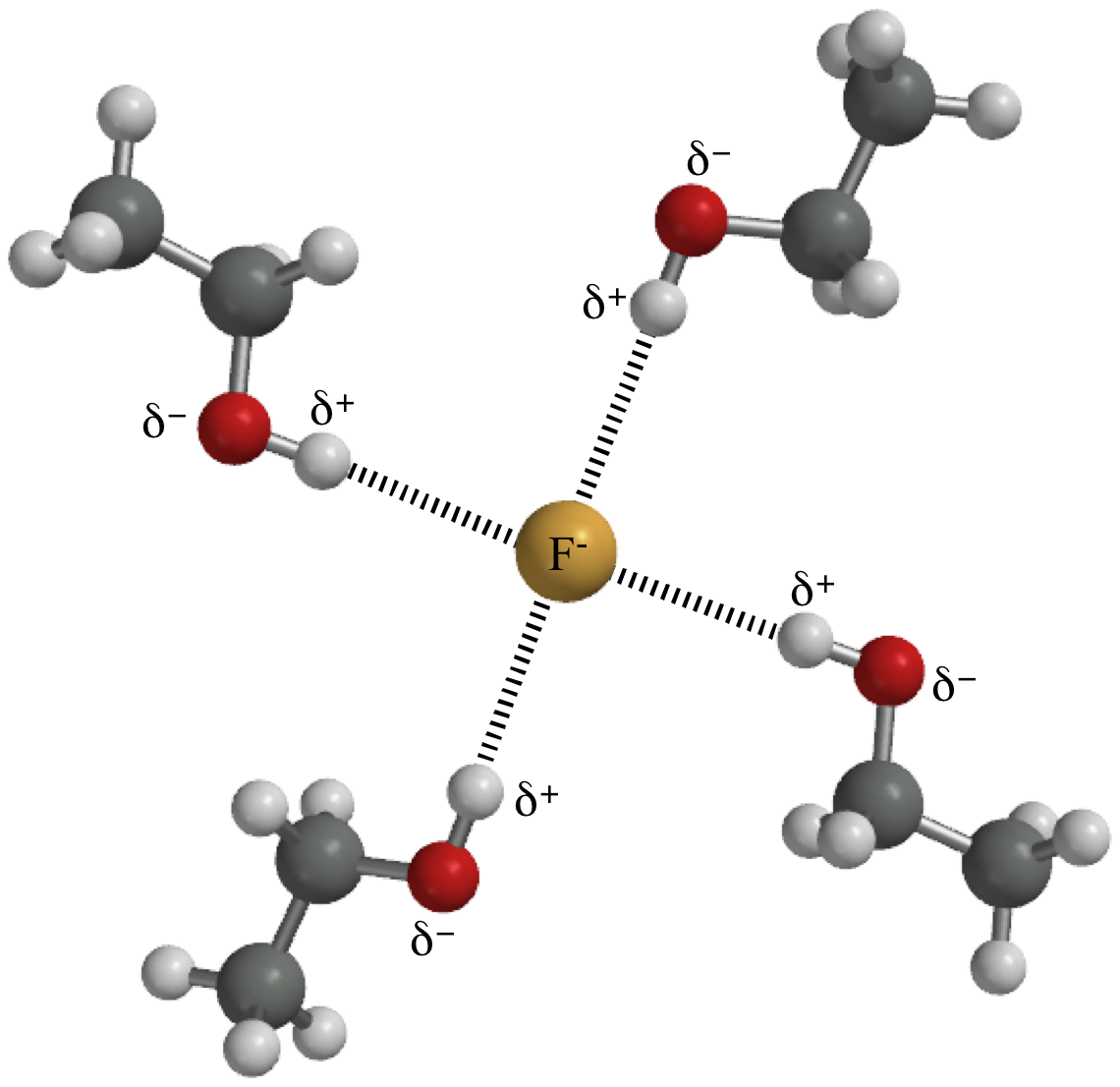
| Hydrogen bonding between fluoride ion and ethanol. | |
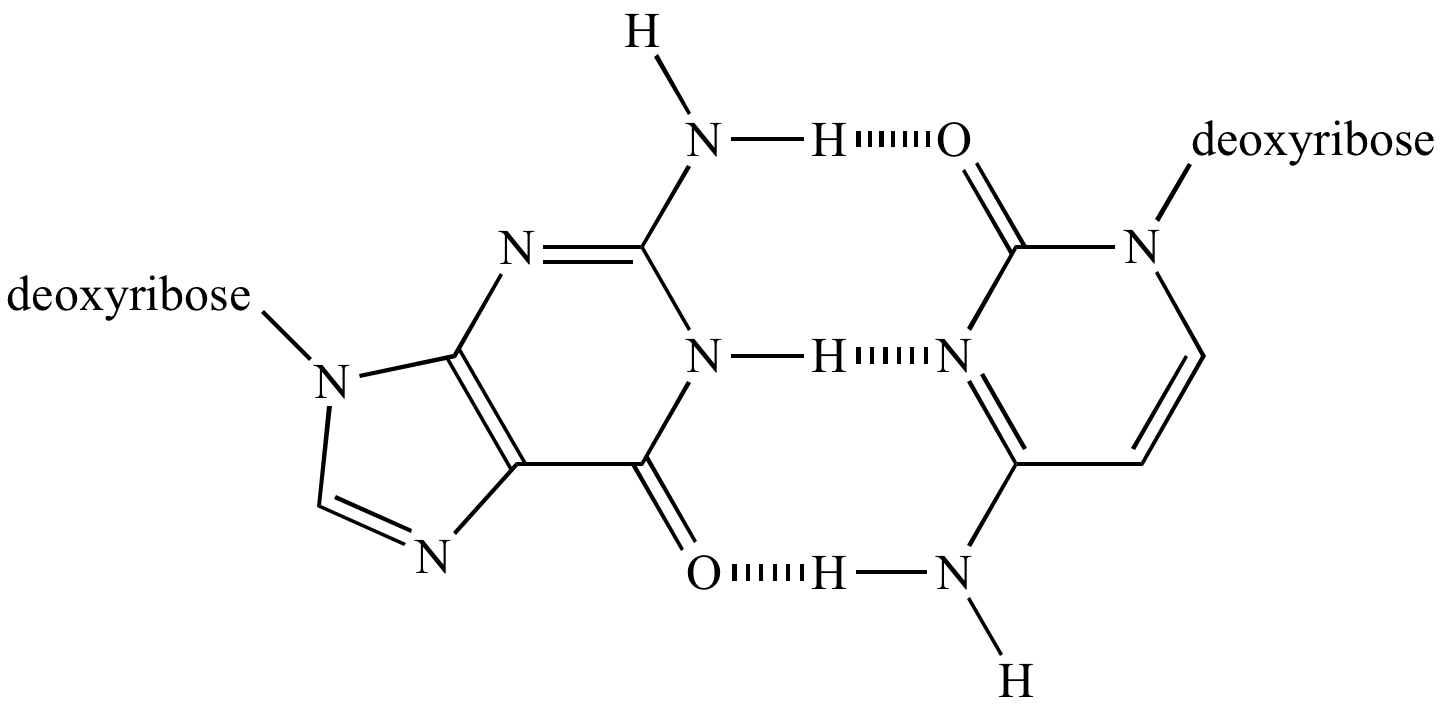
Hydrogen bonding in the guanine-cytosine DNA base pair. |