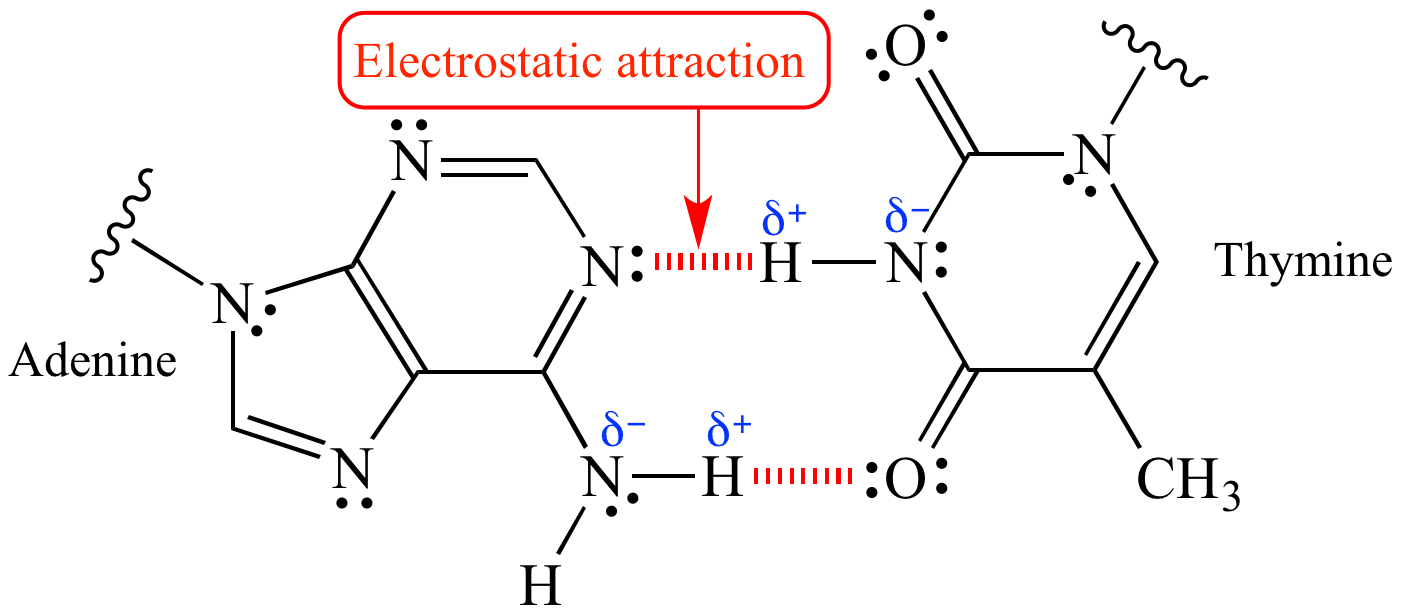
Electrostatic attraction (shown in red) between the δ+ and δ- ends of a polar covalent N-H bond allow for hydrogen bonding and base pairing within the DNA double helix.
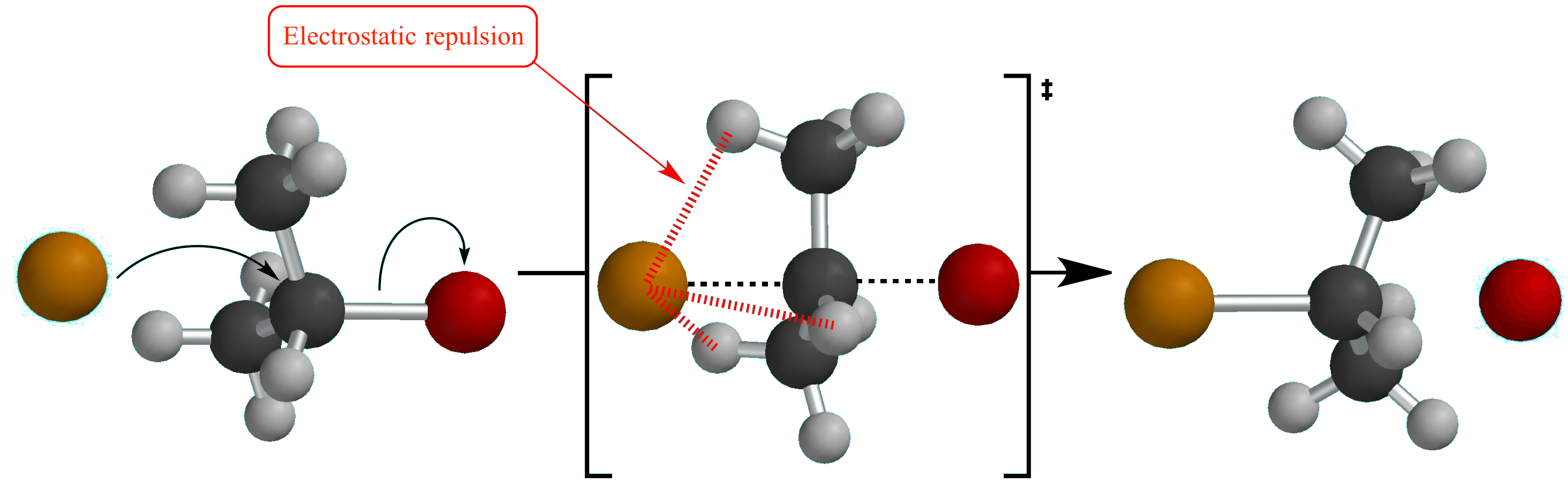
Electrostatic repulsion (also called van der Waals repulsion) between electron clouds is the cause of steric hindrance in an SN2 reaction transition state.