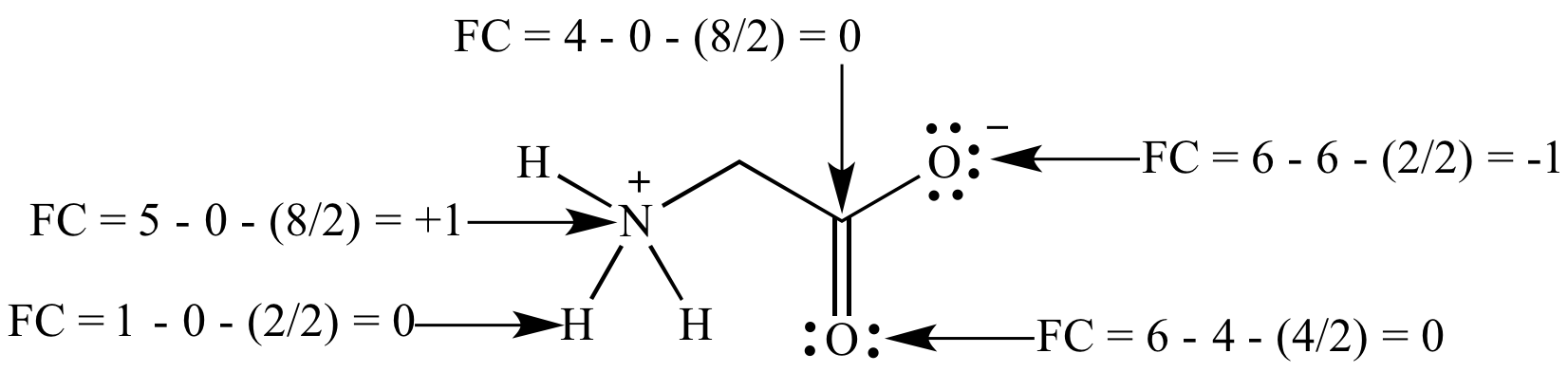
Formal charge: The charge on
an atom in a
Lewis
structure if the
bonding
was perfectly
covalent
and the atom has exactly a half-share of the
bonding
electrons. (The difference between the number of electrons 'owned'
by a
covalently
bonded atom versus the same atom without any
bonds,
i.e., a free atom of the same element.) Calculated using the
formula FC = V - L - (C/2), where: FC = formal charge, V =
number of
valence
electrons for the atom as a free element, L = number of
electrons in
lone
pairs, and C = number of electrons in
covalent
bonds.