Kinetic
control: A reaction in which the product
ratio is determined by the rate
at which the products
are formed.
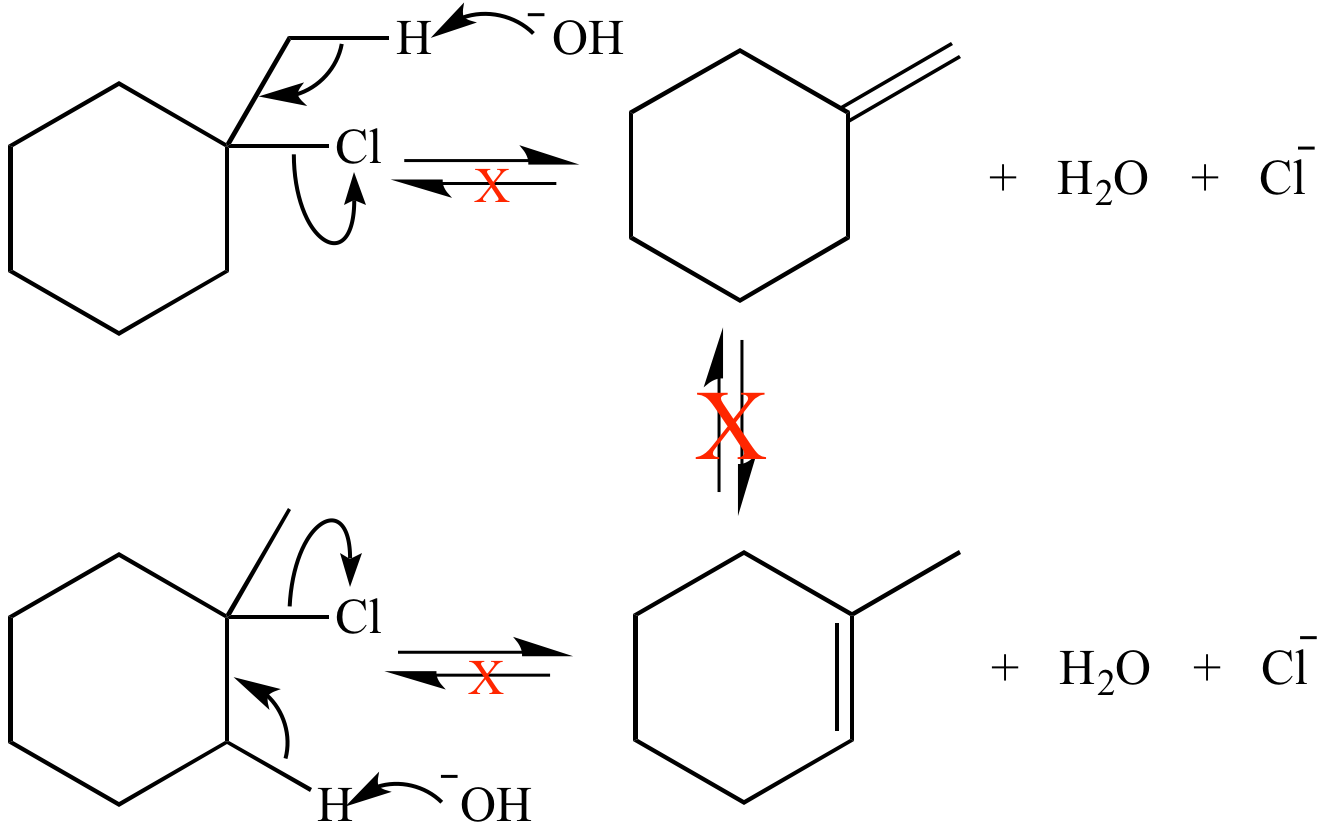
This E2 reaction is irreversible. The alkene products are not in equilibrium, so their relative stability does not control the amount of each product produced. Instead, the relative reaction rates control how much of each product is formed. This reaction is under kinetic control.
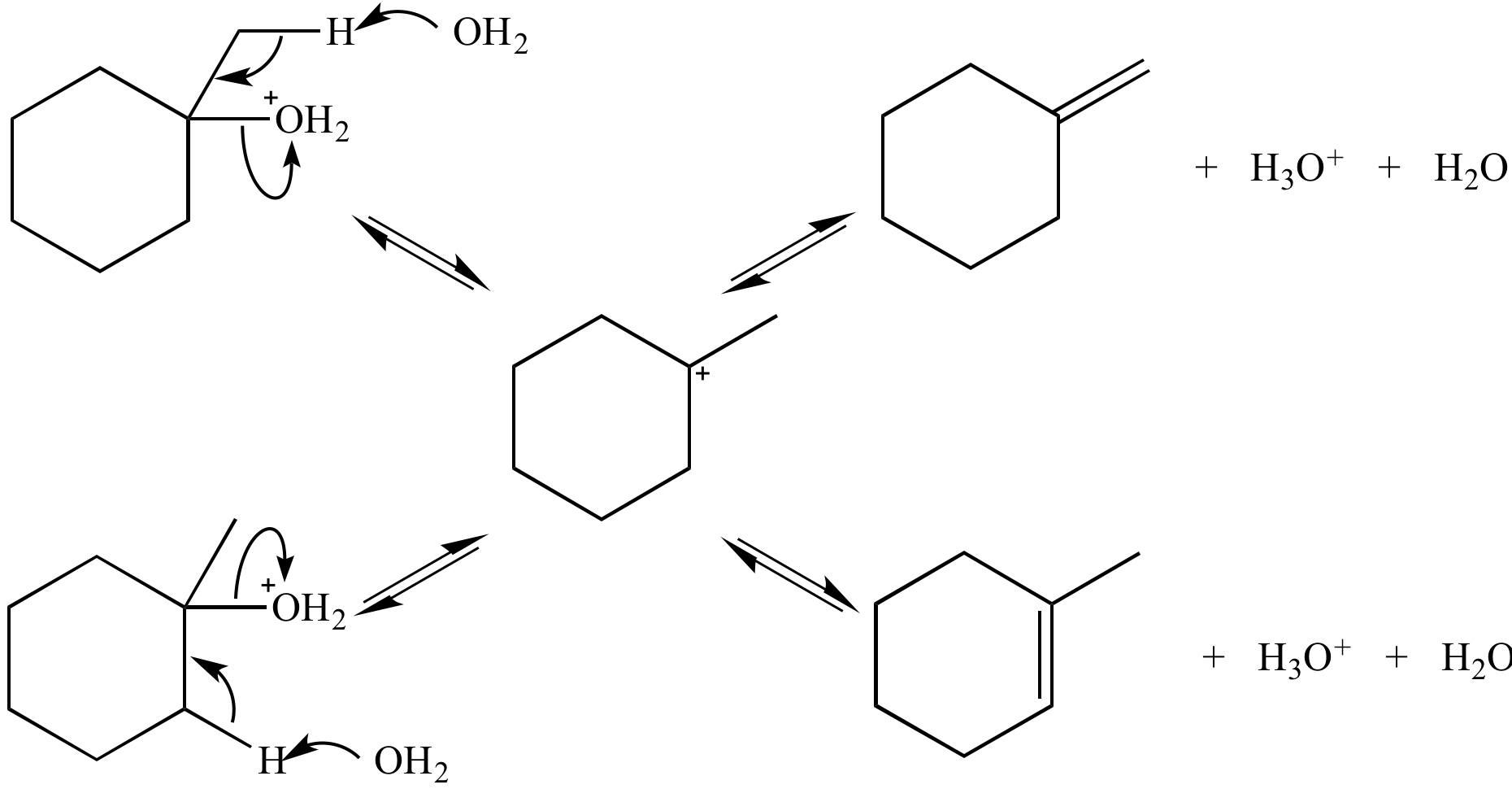
In this E1
reaction the alkene
products
are in equilibrium
(via the carbocation),
so
their relative stability (not the reaction
rates) controls the amount of each product
formed. This reaction is under thermodynamic control.