Equilibrium
(chemical equilibrium): Although the term can have
many meanings, in general, simplified usage
in chemistry the term refers to a system that has two or more
substances (or the same substance in different physical states)
whose concentrations change with time. A system such as a chemical
reaction is said to be at equilibrium when there is no further
change in concentrations over time.
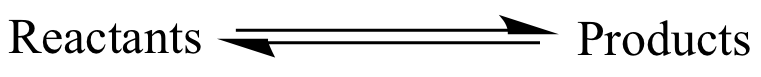
A pair of parallel, opposing
arrows
is used to indicate that
reactants
and
products
are part of an equilibrium. The
arrows
do not necessarily mean that equilibrium has been achieved (i.e.,
that the concentrations are static).