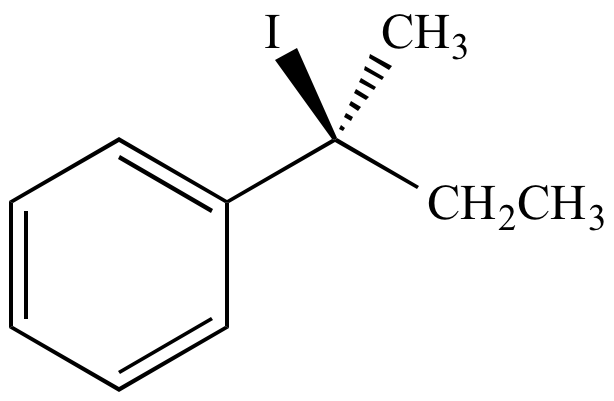
H2O
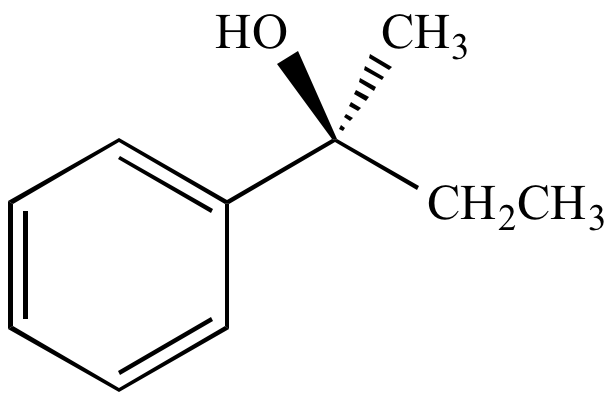
From retention of configuration
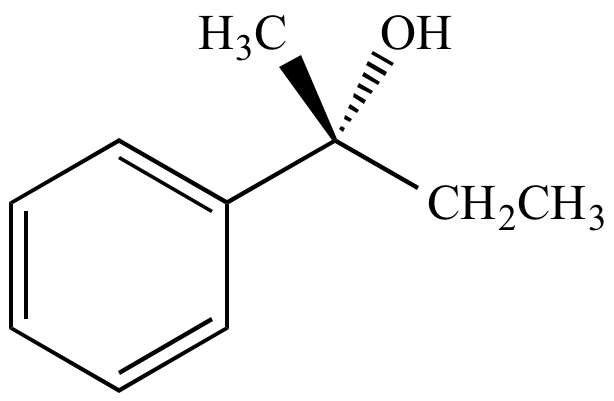
From inversion of configuration
 |
H2O |
 |
+ |
 |
| (S)-2-iodo-2-phenylbutane | (S)-2-phenyl-2-butanol From retention of configuration |
(R)-2-phenyl-2-butanol From inversion of configuration |
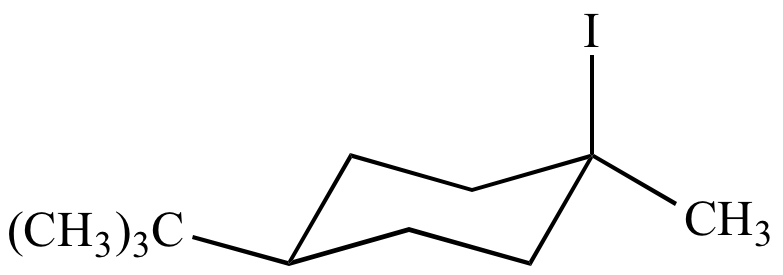 |
H2O |
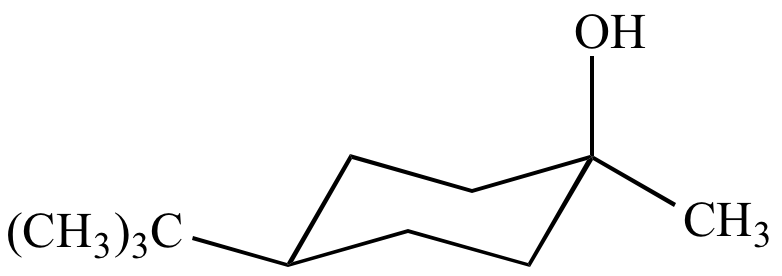 |
+ |
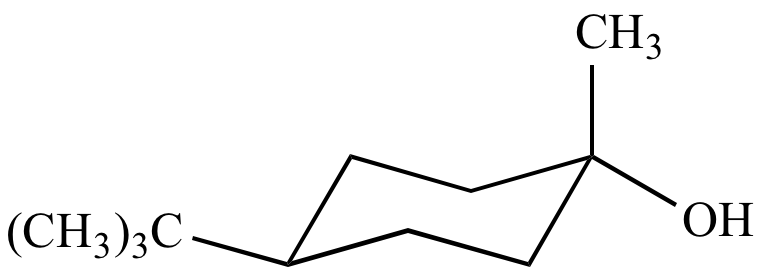 |
| Cis-4-tert-butyl-1-iodo-1-methylcyclohexane | Cis-4-tert-butyl-1-methylcyclohexanol From retention of configuration |
Trans-4-tert-butyl-1-methylcyclohexanol From inversion of configuration |