 |
 |
|
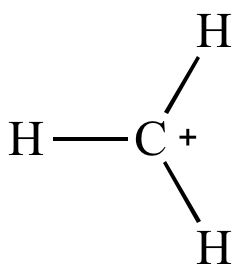
| Methyl
carbocation Less stable |
|
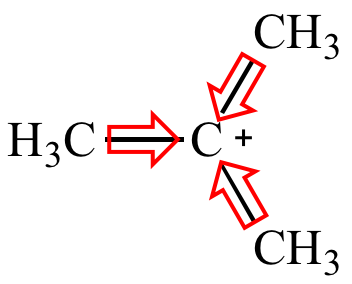
Tert-butyl carbocation More stable |
 |
 |
|
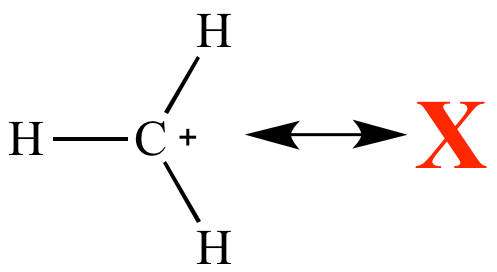
| Carbocation
without resonance Less stable |
|
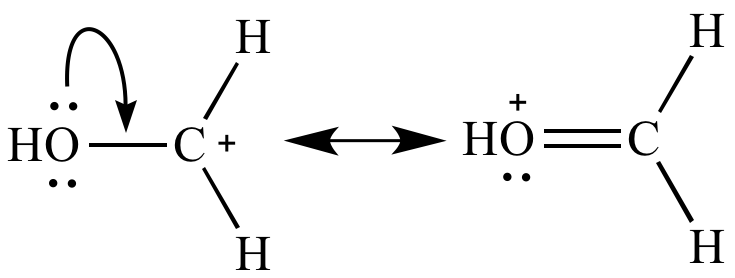
Carbocation
with resonance More stable |