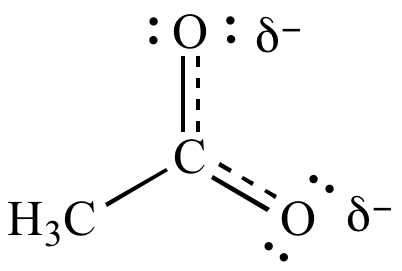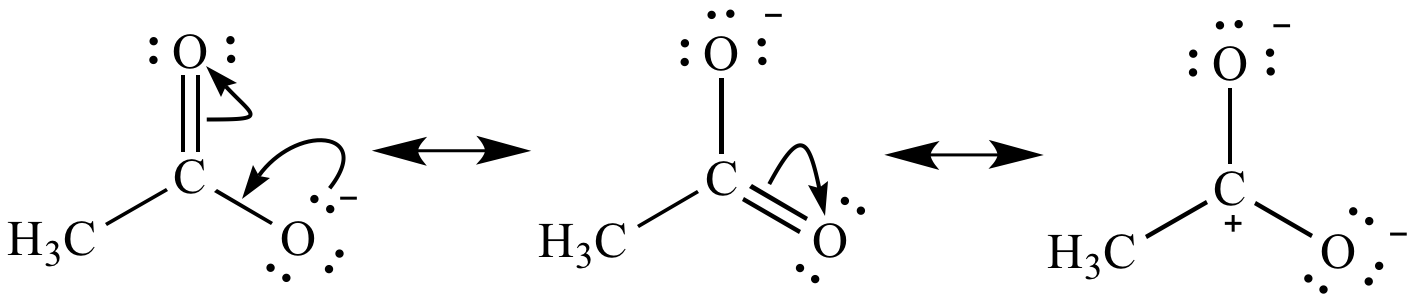
Acetate ion resonance contributors. The first two contributors are equally significant (they don't violate any of the resonance contributor preference rules). The third contributor is not significant because it violates three resonance contributor preference rules. Non-significant resonance contributors are usually not included in the resonance hybrid (shown below).