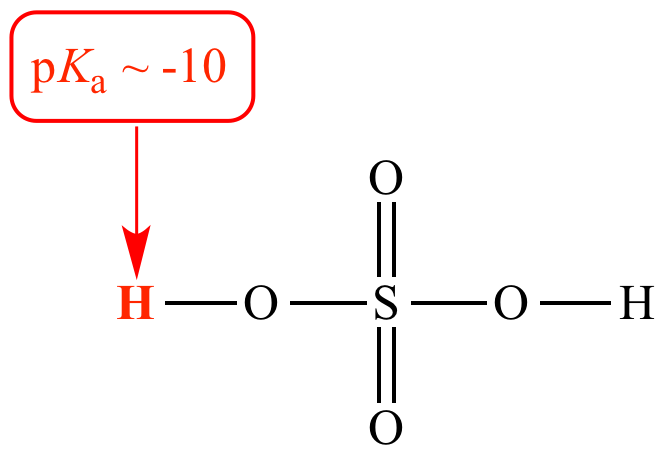
Sulfuric acid (H2SO4) is a strong Bronsted acid (pKa -10). When sulfuric acid is under discussion it is called an acid or a Bronsted acid.

Aluminum chloride (AlCl3) is a Lewis acid because the aluminum atom has an open valence shell. When aluminum chloride is under discussion it is called a Lewis acid or an electrophile.