 |
|
 |
 |
 |
|
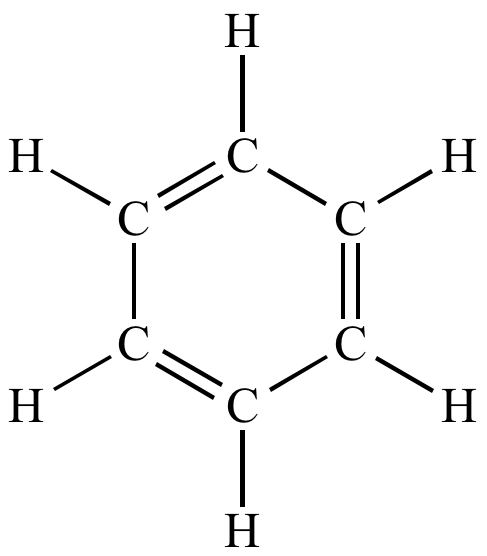
| A typical Kekule structure. Does not include lone pairs. |
|
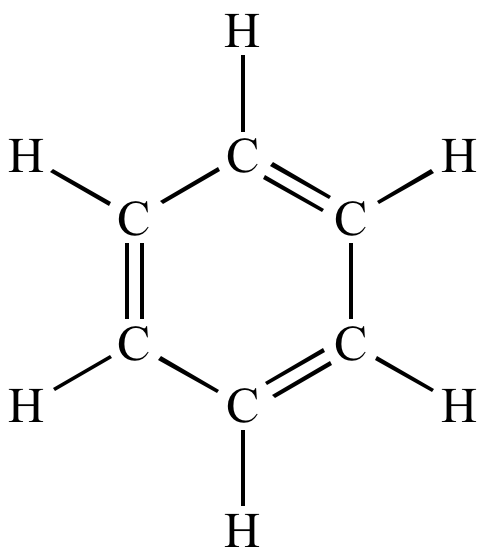
Corresponding Lewis
structure. Lone pairs shown. |
 |
|
 |
 |
 |
|
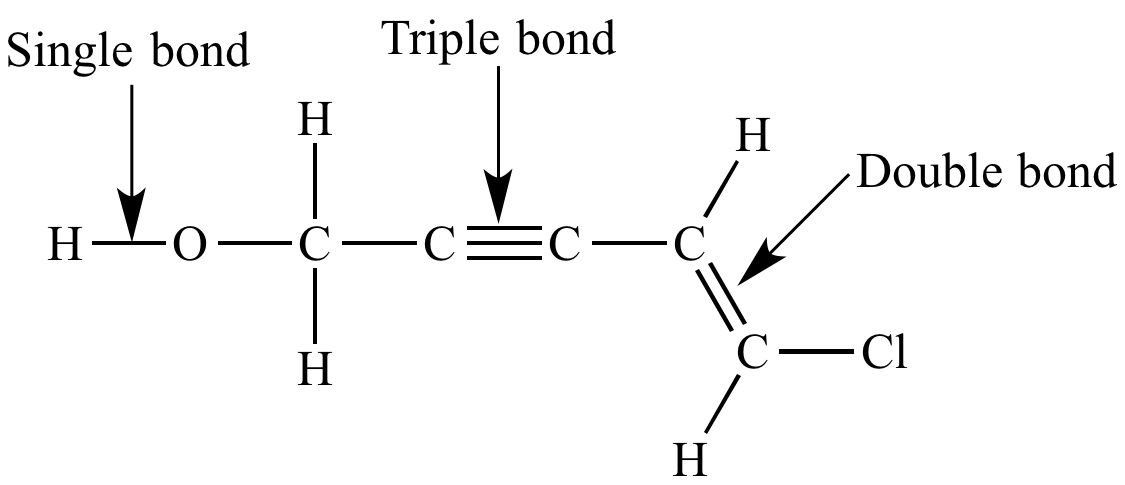
| A typical Kekule structure. Does not include lone pairs. |
|
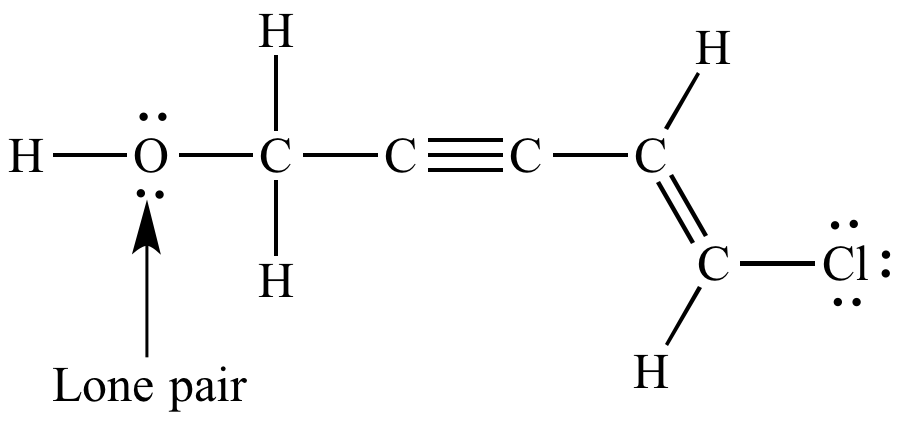
Corresponding Lewis
structure. Lone pairs shown. |