Na+ -OCH2CH3
CH3CH2OH, heat
- N2
CH3CH2OH
 |
H2NNH2 |
 |
Na+ -OCH2CH3 CH3CH2OH, heat - N2 |
 |
CH3CH2OH |
 |
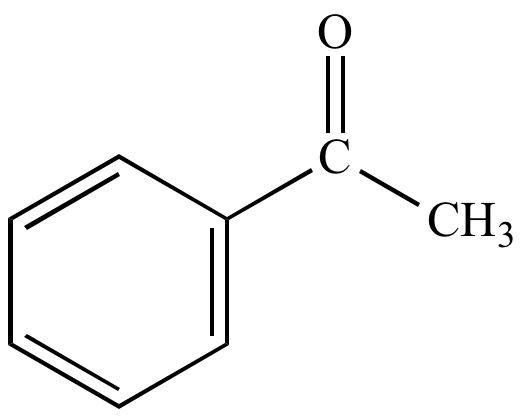
| Acetophenone |
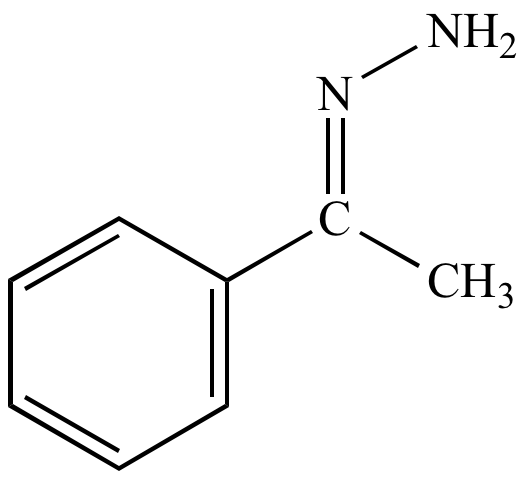
Acetophenone
hydrazone |
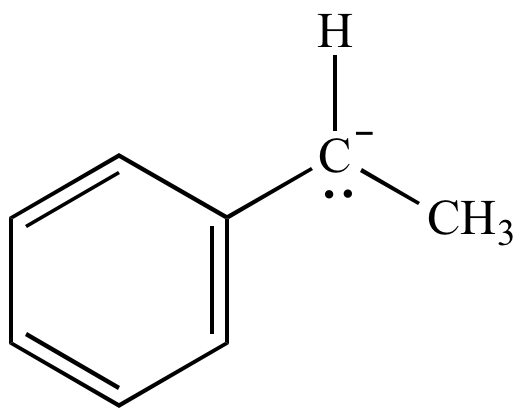
Carbanion
intermediate |
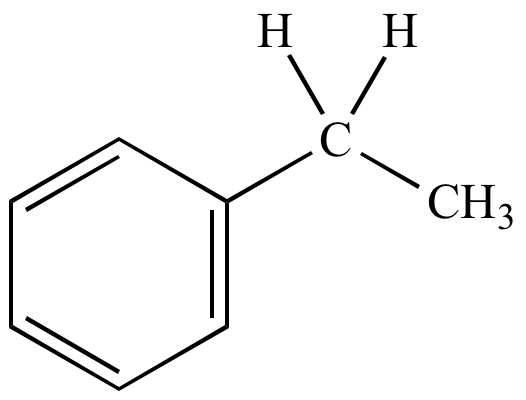
Ethylbenzene |