SN2 reaction of triphenylphosphine (Ph3P) with iodomethane (CH3I) produces methyl triphenylphosphonium iodide (Ph3PCH3+ I-), a phosphonium salt.
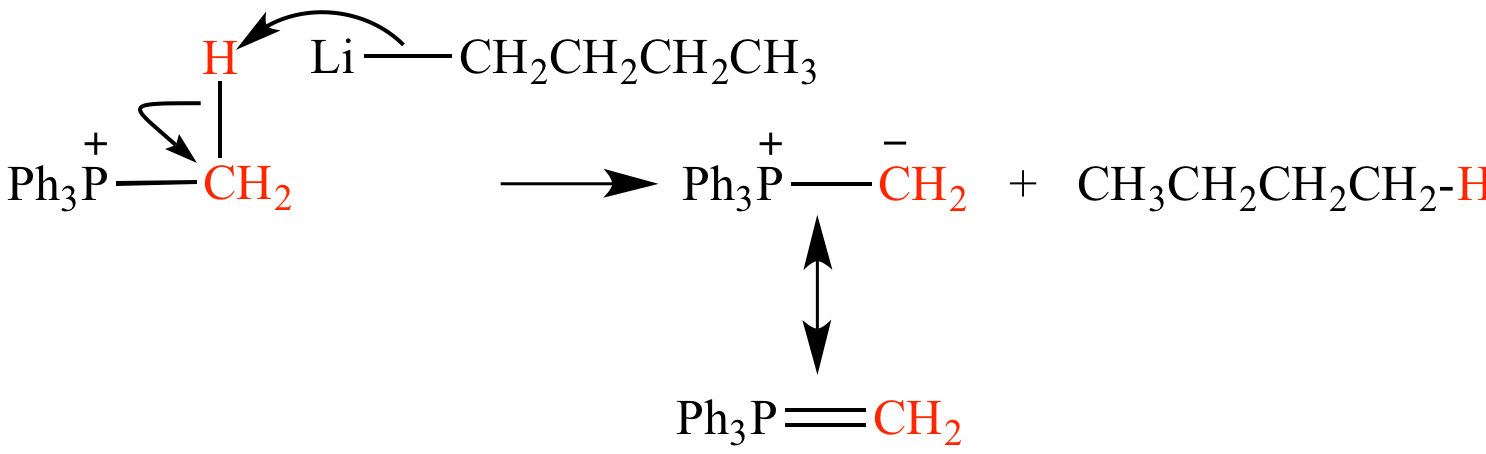
Phosphonium salt deprotonation by butyllithium (CH3CH2CH2CH2Li) forms methyl triphenylphosphonium ylide (Ph3P=CH2) and butane (CH3CH2CH2CH3).
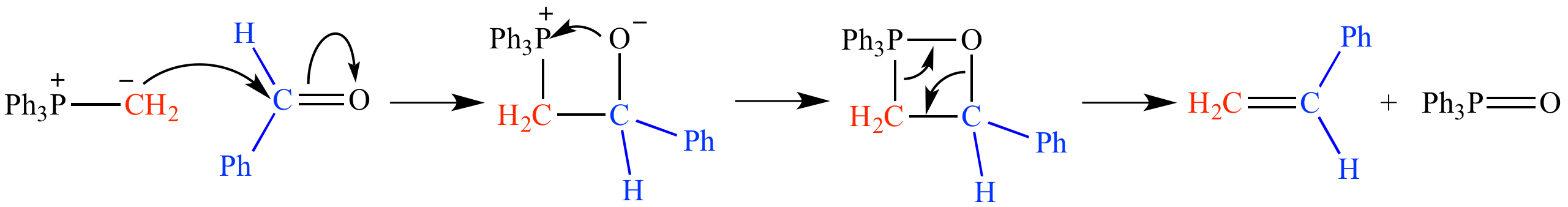
The phosphonium ylide adds to benzaldehyde to produce styrene (PhCH=CH2), along with triphenylphosphine oxide (Ph3P=O) as a by-product. The four-membered ring intermediate is an oxaphosphetane.