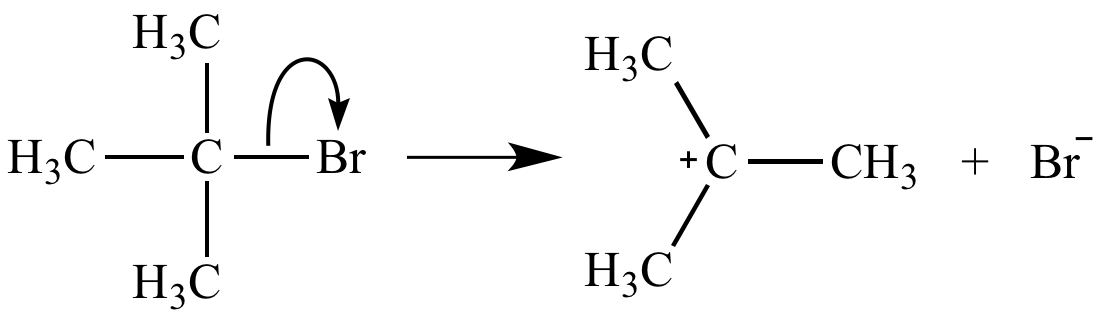
Ionization of a carbon-leaving group bond, the rate-determining step of an SN1 reaction, is unimolecular. Its rate equation is rate = k [(H3C)3CBr].
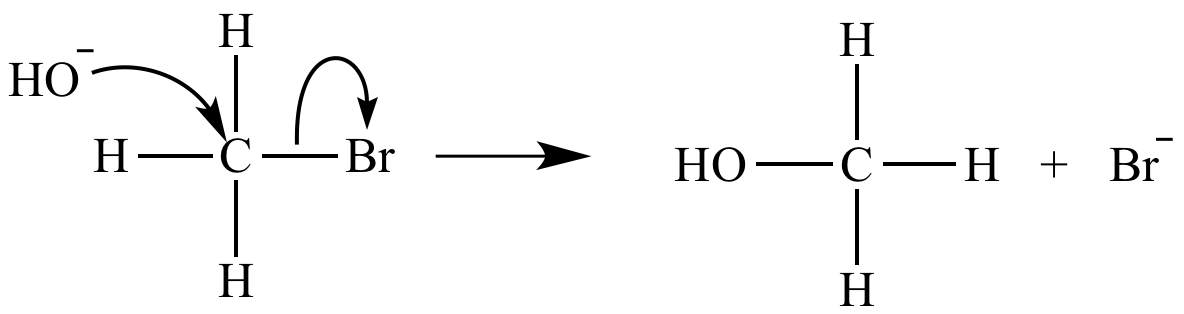
The rate-determining step of an SN2 reaction is bimolecular. Its rate equation is rate = k [CH3Br] [HO-].
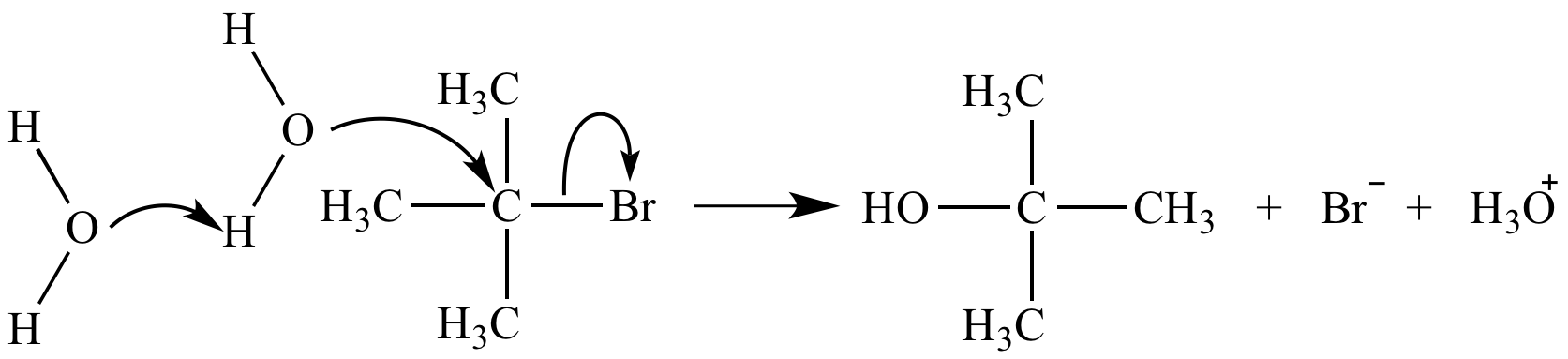
A termolecular mechanism step is nearly impossible due to the very low probability of simultaneous collision of three reactants. The termolecular substitution reaction mechanism shown here does not occur. The actual mechanism (SN1) involves three steps.