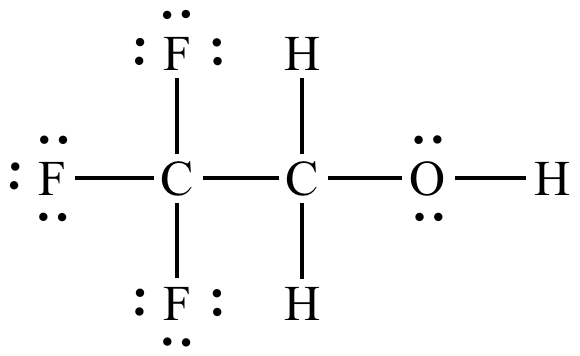
Lewis structure for trifluoroethanol.
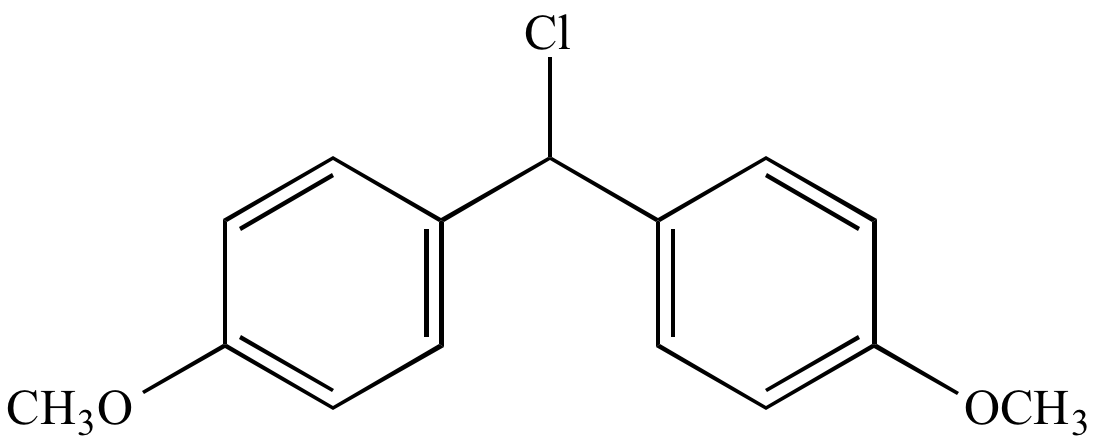 |
CF3CH2OH |
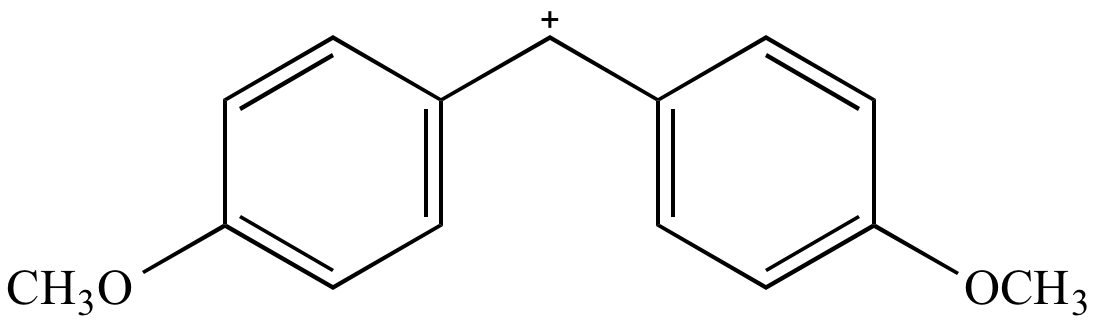 |
Slow CF3CH2OH |
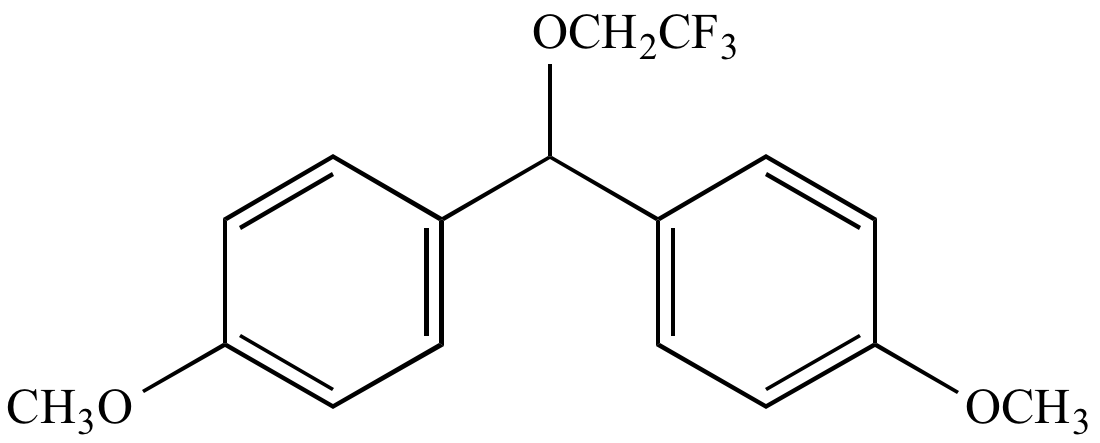 |
| Molecule
A |
Carbocation
intermediate
(reddish-orange) |
Solvolysis
product |
 |
CH3CH2OH |
 |
Fast CH3CH2OH |
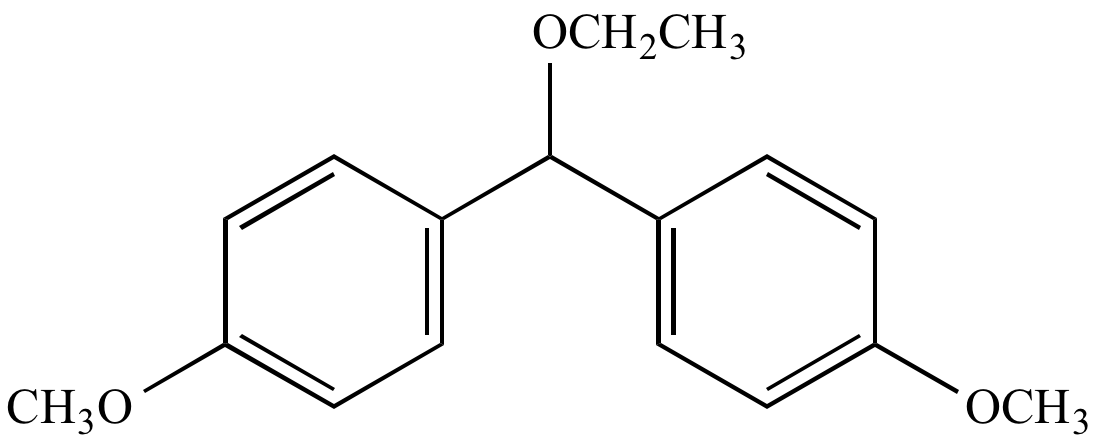 |
| Molecule
A |
Carbocation
intermediate
(reddish-orange) |
Solvolysis
product |