Thermodynamic
control:
A reaction in which the product
ratio is determined by the relative stability of the products.

This E2 reaction is irreversible. The alkene products are not in equilibrium, so their relative stability does not control the amount of each product produced. Instead, the relative reaction rates control how much of each product is formed. This reaction is under kinetic control.
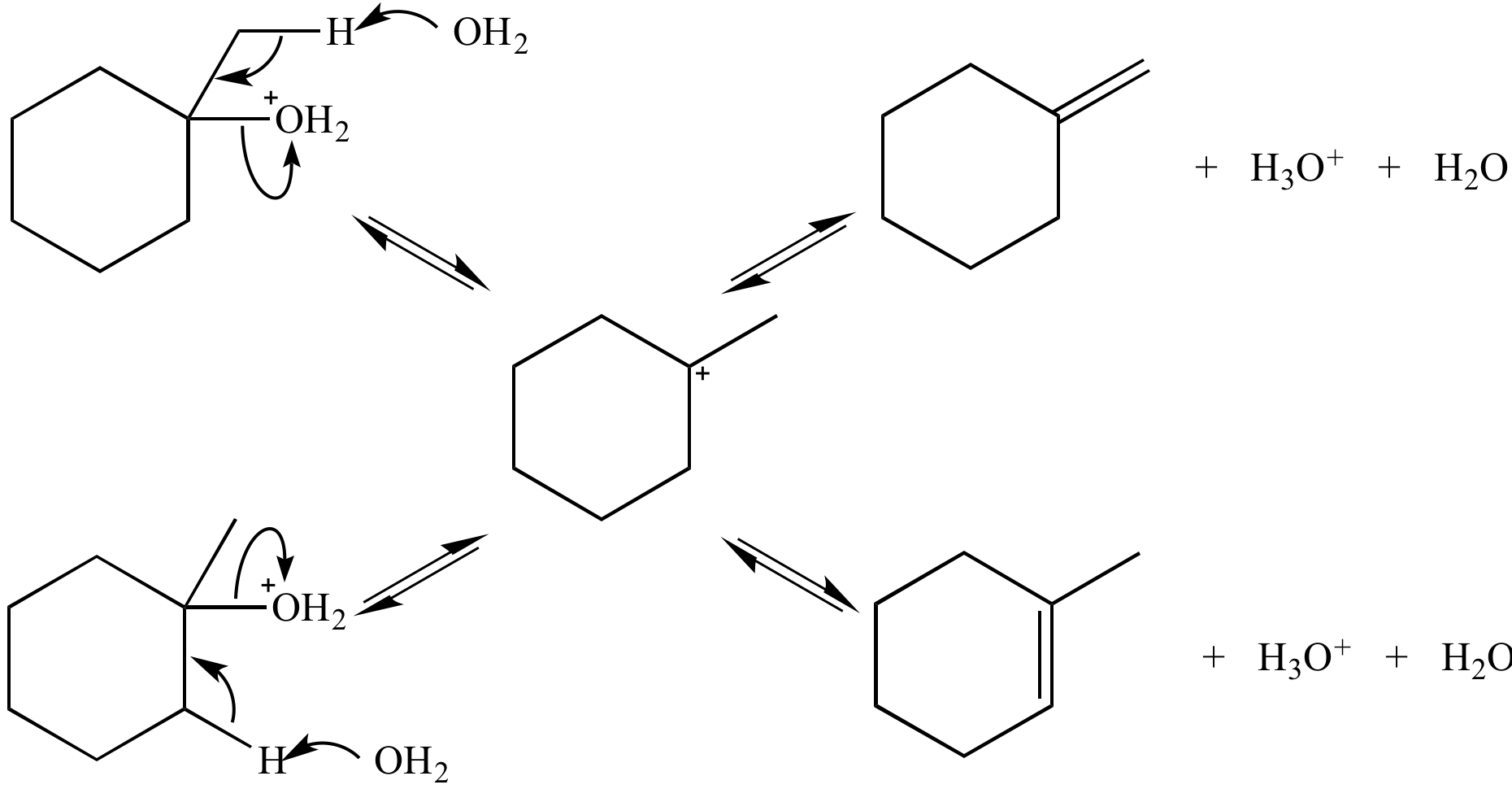
In this E1 reaction the alkene products are in equilibrium (via the carbocation), so their relative stability (not the reaction rates) controls the amount of each product formed. This reaction is under thermodynamic control.