Stoichiometry:
The study of the relationship between quantity of reactants
and products
in a chemical reaction.
In this SN2
reaction, one mole
of CH3Cl (molar
mass = 50.49 g mol-1)
reacts with one mole
of NaI (molar
mass = 149.89 g mol-1)
to produce
one mole
of CH3I (molar
mass = 141.95 g mol-1)
plus one mole
of NaCl (molar
mass = 58.44 g mol-1).
From
this we can deduce that 29.7 g of NaI (0.198 mole)
are required to completely react with 10.0 g (0.198 mole)
of CH3Cl and that this reaction will produce 28.1 g
(0.198 mole)
of CH3I and 11.6 g (0.198 mole)
of NaCl.
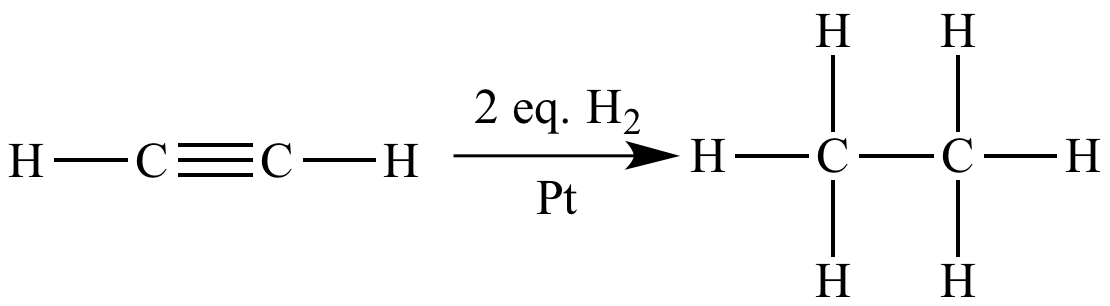
In this catalytic
hydrogenation reaction, one mole
of acetylene
(molar
mass = 26.04 g mol-1)
reacts with two moles
of H2 (molar
mass = 2.016 g mol-1)
and a catalytic
amount of Pt to produce
one mole
of ethane
(molar
mass = 30.07 g mol-1).
From
this we can deduce that 20.0 liters of H2 gas (1.800
g; 0.893 mole
under standard
conditions) are required to completely react with 10.0
liters of acetylene
(11.6 g; 0.446 mole
under standard
conditions), and that this reaction will produce
10.0 liters of ethane
(13.4 g; 0.446 mole
under standard
conditions). Pt is the catalyst
and therefore not consumed in the reaction. The amount of Pt
present controls the reaction
rate, but not the reaction stoichiometry.