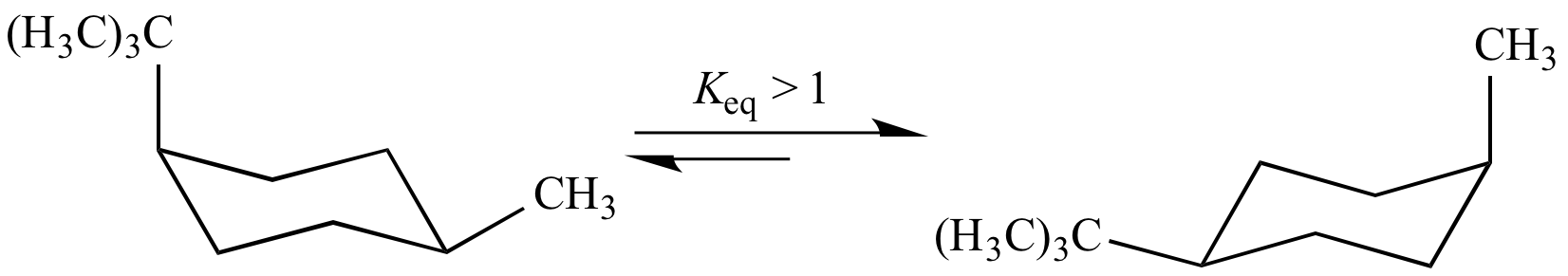
In this cyclohexane chair flip equilibrium, Keq > 1. This is an example of a steric effect caused by van der Waals repulsion: the tert-butyl group is larger (and therefore causes more van der Waals repulsion) than the methyl group.
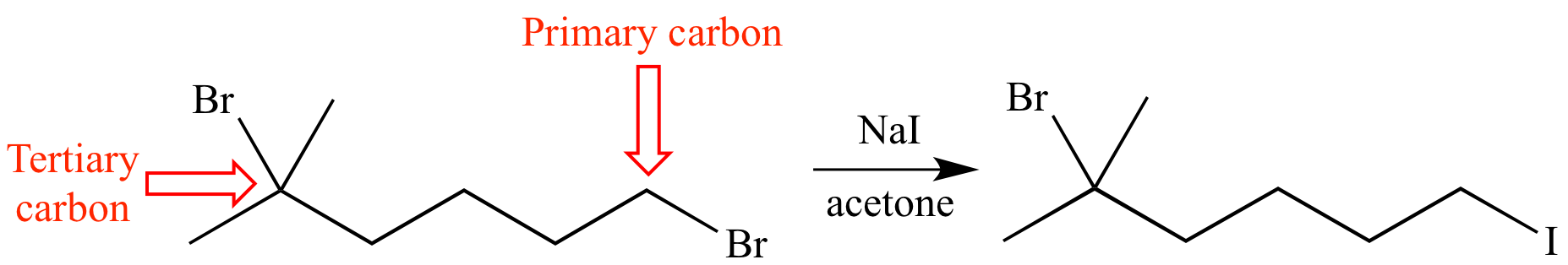
This SN2 reaction occurs at a primary carbon but not a tertiary carbon. This is an example of a steric effect caused by steric hindrance: the tertiary carbon is more hindered than the primary carbon.