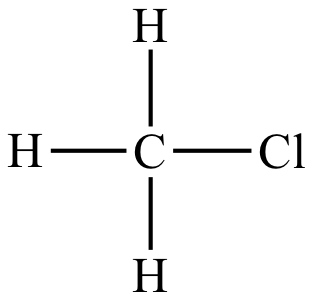
 |
|
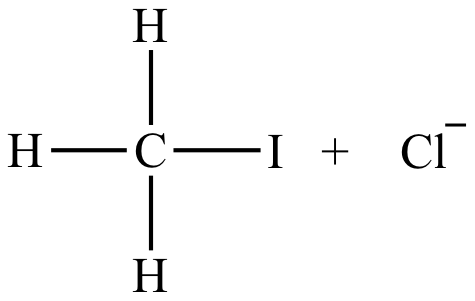 |
|
Rate = k [CH3Cl] [I-] |
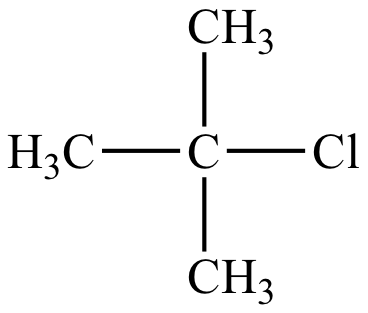 |
|
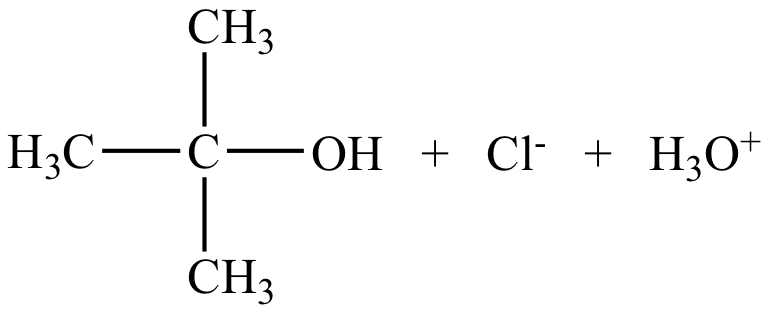 |
|
Rate = k [(CH3)3CCl] |
 |
|
 |
|
Rate = k [CH3Cl] [I-] |
 |
|
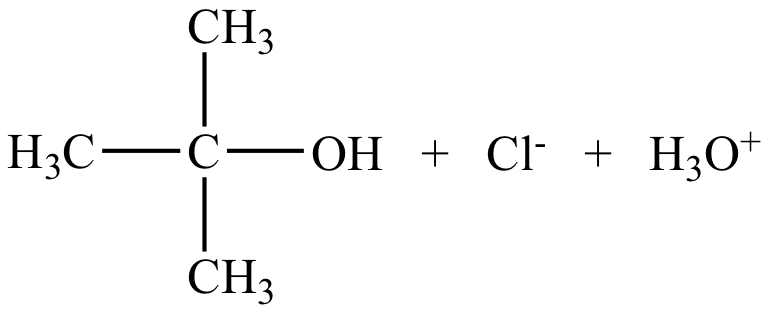 |
|
Rate = k [(CH3)3CCl] |