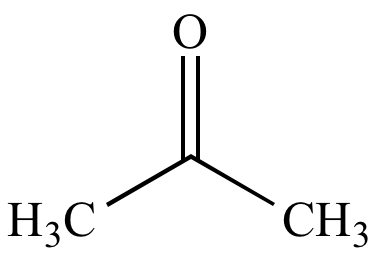
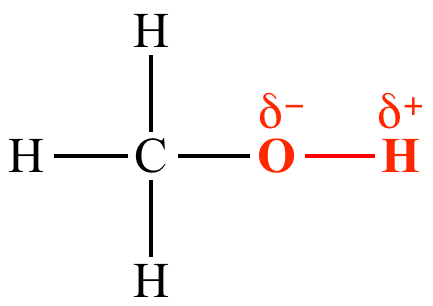Proticity:
Characterization of
a substance as
protic (a
hydrogen
bond
donor) or
aprotic
(incapable of
donating
a
hydrogen bond). Not to be confused with proticity in
biology,
which is an electrical current generated by a flow of
protons
(instead of a flow of electrons).