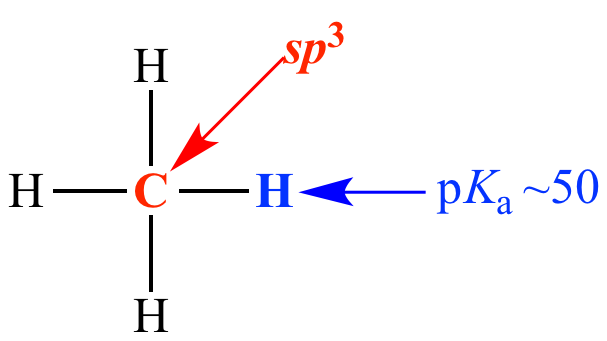
Carbon is sp3 (25% s character)
pKa ~ 50
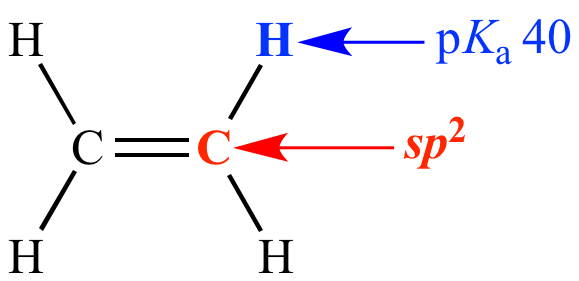
Carbon is sp2 (33% s character)
pKa = 40
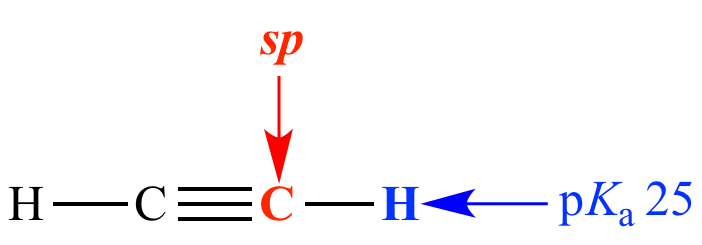
Carbon is sp (50% s character)
pKa = 25
 |
Methane Carbon is sp3 (25% s character) pKa ~ 50 |
 |
Ethylene Carbon is sp2 (33% s character) pKa = 40 |
 |
Acetylene Carbon is sp (50% s character) pKa = 25 |