 |
 |
 |
||
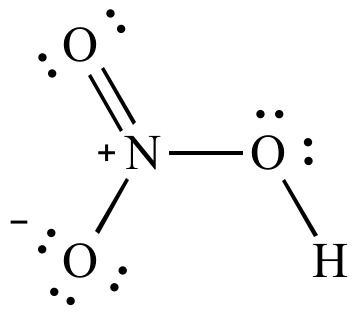
| Lewis
structure |
|
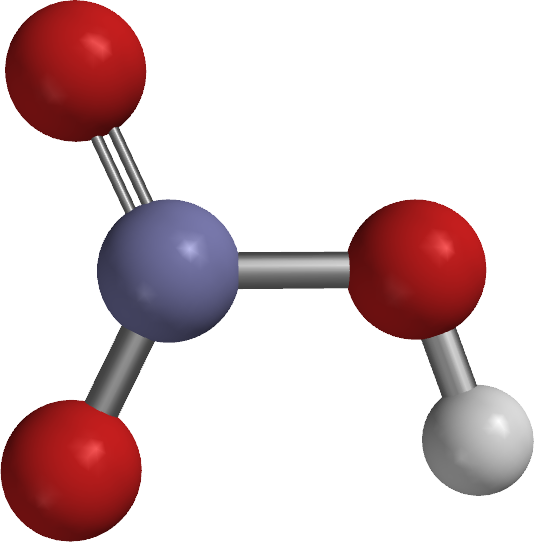
Ball and spoke model |
|
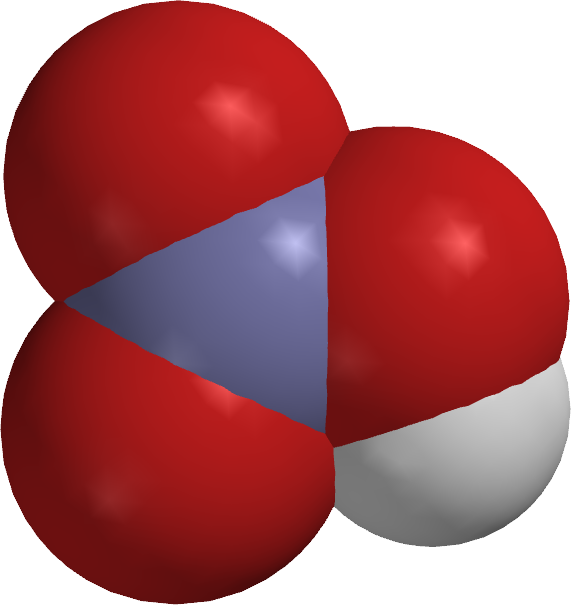
Space
filling
model |
 |
 |
 |
||
| Lewis
structure |
|
Ball and spoke model |
|
Space
filling
model |