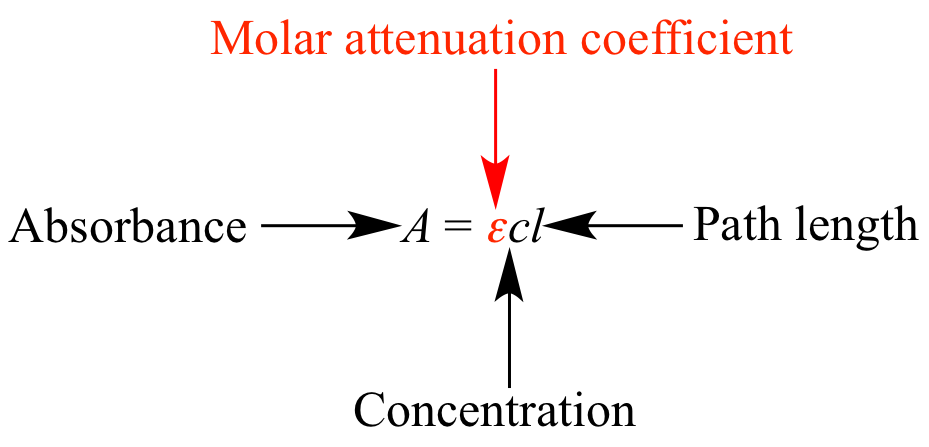
Molar
attenuation coefficient (molar absorptivity; molar extinction
coefficient): The degree to which a solution absorbs
light, in terms of the solution's concentration. Represented by
ε
in the
Beer-Lambert
law. Greater
ε
= more light absorbed per
mole
of
solute.