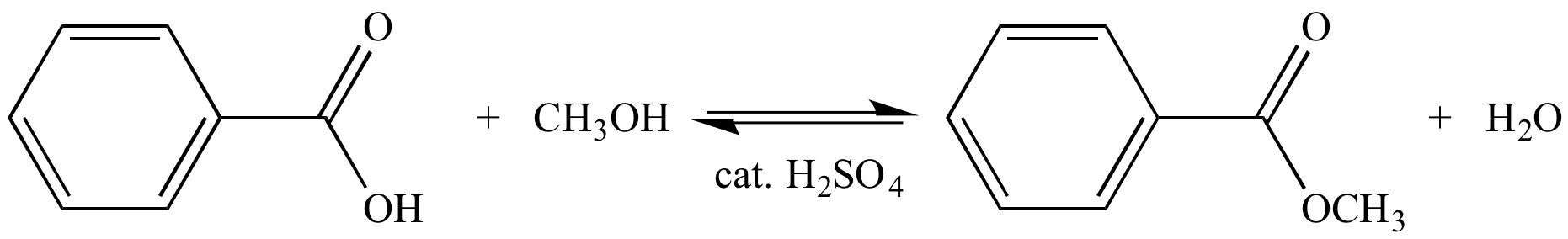
Heating a 1:1 mixture of benzoic acid and methanol with a catalytic amount of sulfuric acid produces an ester and water. At equilibrium, the concentrations of the reactants and products are about equal, and Keq ~ 1. This is not a very efficient way to transform a carboxylic acid into an ester.
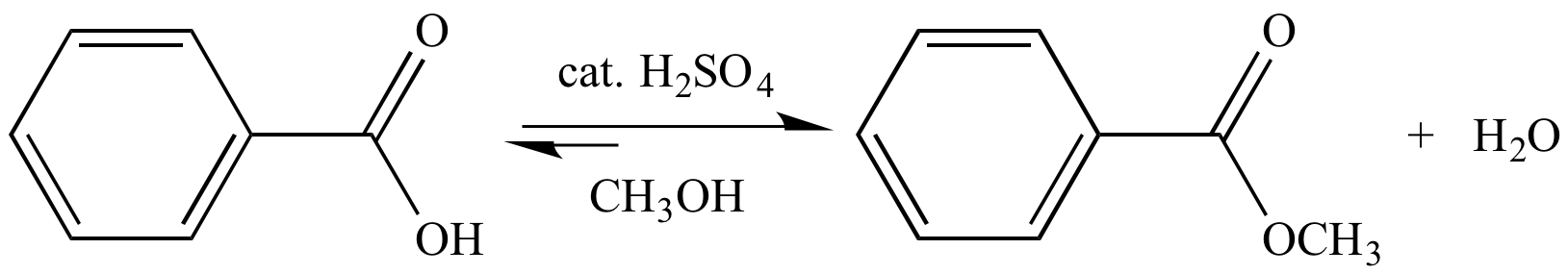
The Fischer esterification reaction takes advantage of Le Chatelier's principle to increase the amount of carboxylic acid that is esterified. The equilibrium is shifted towards products by using a large excess of the alcohol (it is used as the reaction solvent), and (in some cases) also removing water as it it formed.