 |
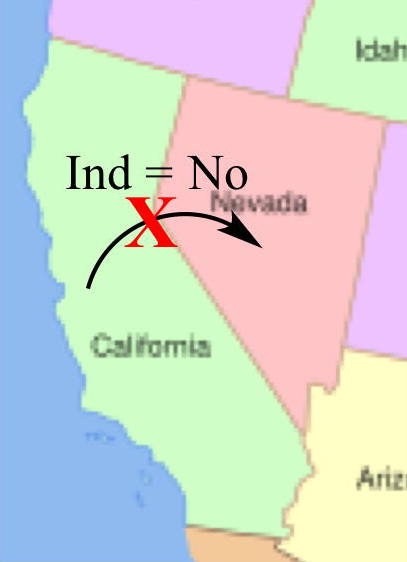
Without an IND application,
an investigational drug
cannot be shipped across state lines (for example from
California to Nevada). |
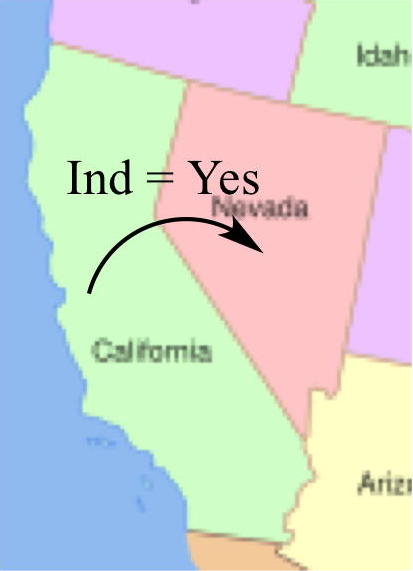 |
With an approved IND
application, an investigational drug
can be shipped across state lines. |