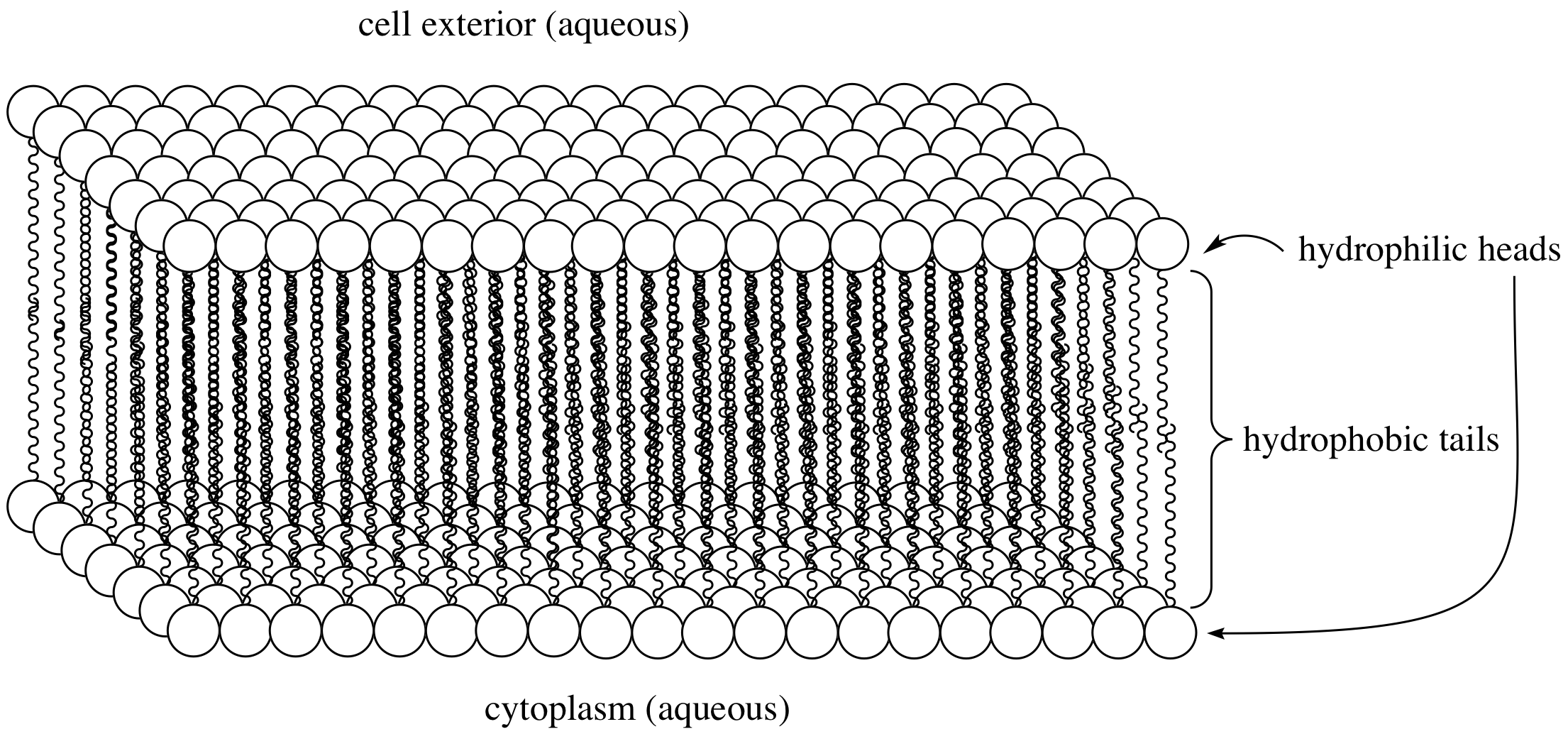
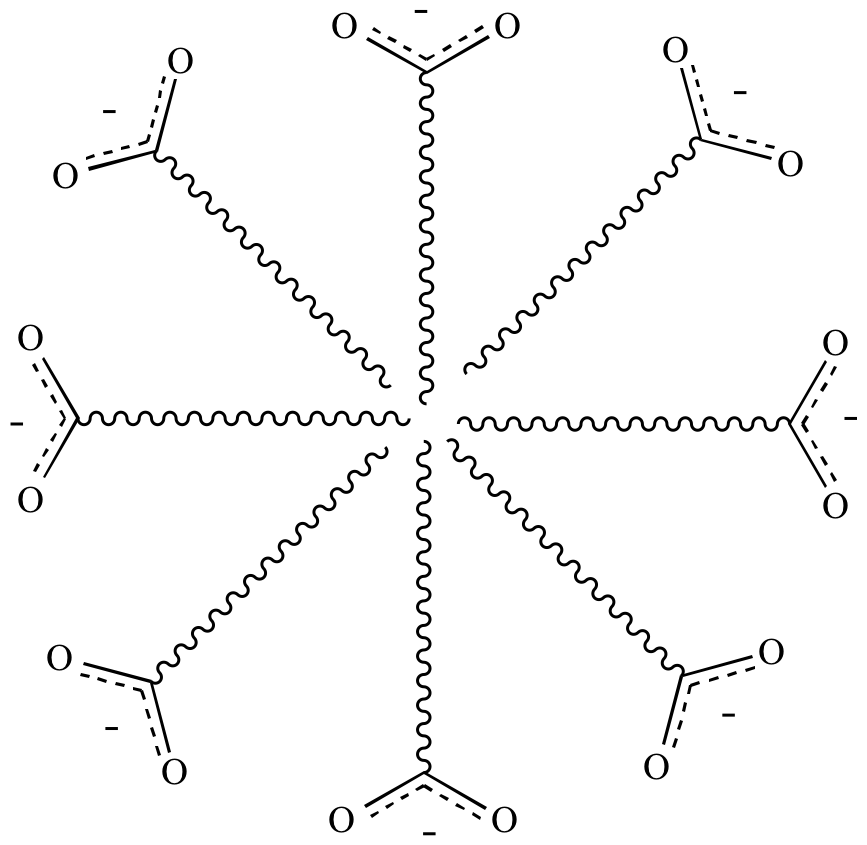
Hydrophobic
effect:
The arrangement of molecules
in a polar
solvent
(such as water)
which causes the polar
regions to be oriented outwards towards the solvent
and the nonpolar
regions oriented inwards away from the polar
solvent.
Can also be thought of as the tendency for water
to exclude nonpolar
(hydrophobic)
molecules.
 |
 |