H-rule
(hydrogen and halogen
rule; hydrogen rule): For a molecule
containing only hydrogen, carbon, oxygen, nitrogen, fluorine,
chlorine, bromine, and iodine, the maximum number of monovalent
atoms possible (max H) for a given number of carbons (C) and
nitrogens (N) is given by the equation max H = 2C + N + 2. Note
this gives the maximum number; less may be present. The rule may
or may not apply if other elements such as sulfur or phosphorous
are present. Often called the Hydrogen Rule, but this is
misleading because the rule gives the maximum number of
hydrogens plus halogens.
 |
 |
 |
||||
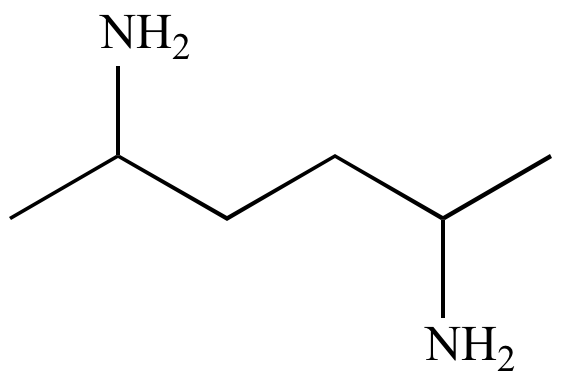
| C6H16N2 |
|
C6H12ClF |
|
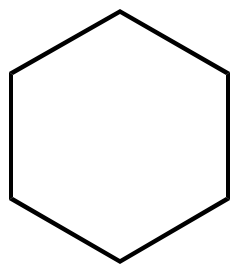
C6H12 |
|
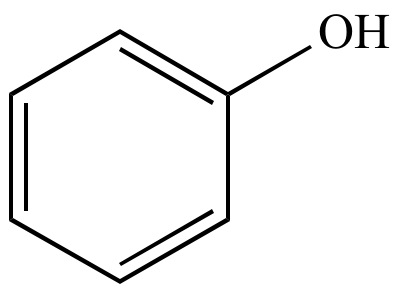
C6H6O |