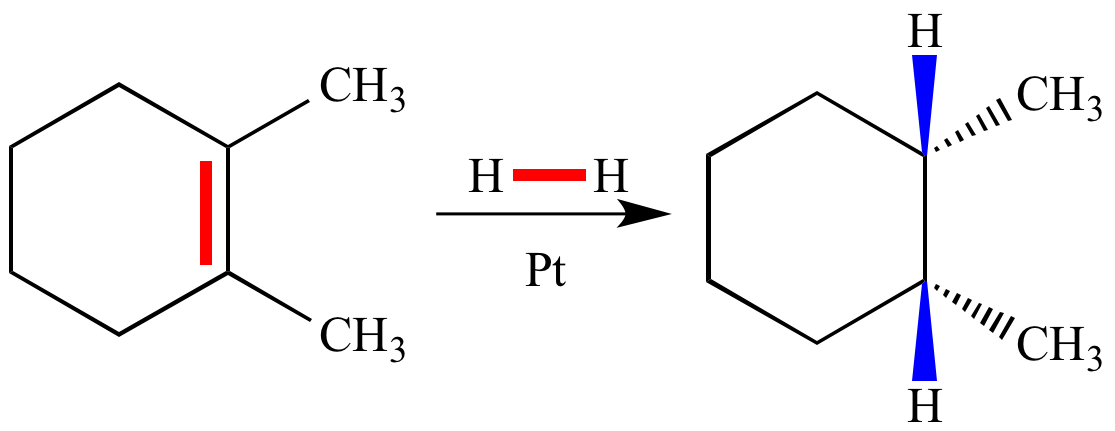
The enthalpy of hydrogenation (ΔH of hydrogenation; also called heat of hydrogenation) for this catalytic hydrogenation reaction can be estimated from (strength of two C-H σ bonds gained) - [(strength of carbon-carbon π bond lost) + (strength of H-H σ bond lost)]. In this example the bonds lost are shown in red, and the bonds gained in blue. The empirical (observed) ΔH is ~ -30 kcal mol-1.