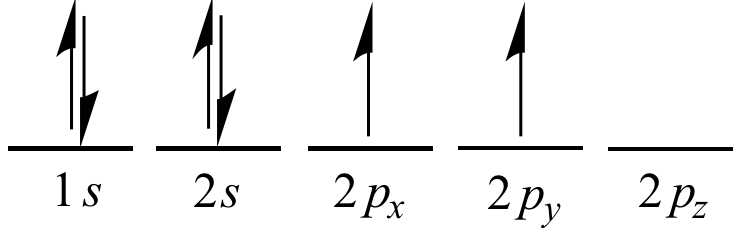Electron
configuration: The arrangement of electrons within an
atom's or
molecule's
orbitals.
The
orbital
designations are italicized, and the number of electrons in each
orbital
is superscripted. For example, the electron configuration of an
isolated (non
bonded)
carbon atom is 1
s2
2
s2
2
p2.
This means the atom's 1
s,
2
s,
and 2
p
orbital
shells are each occupied by two electrons. This electron
configuration can also be written as [He] 2
s2
2
p2,
where [He] represents the core (non
valence
shell) electrons. A more detailed electron configuration
describes the distribution of the electrons within the
p
orbitals: 1
s2
2
s2
2
px1
2
py1.