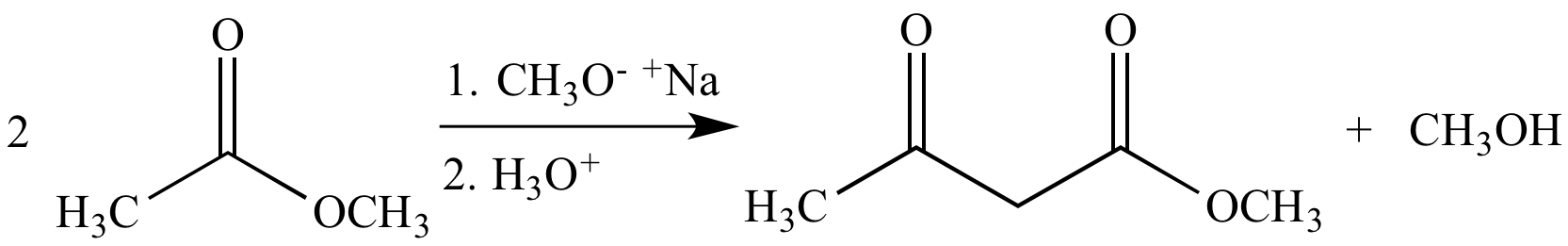
Base-promoted Claisen condensation of methyl acetate gives methyl acetoacetate, a β-keto ester (as a mixture of keto and enol tautomers).
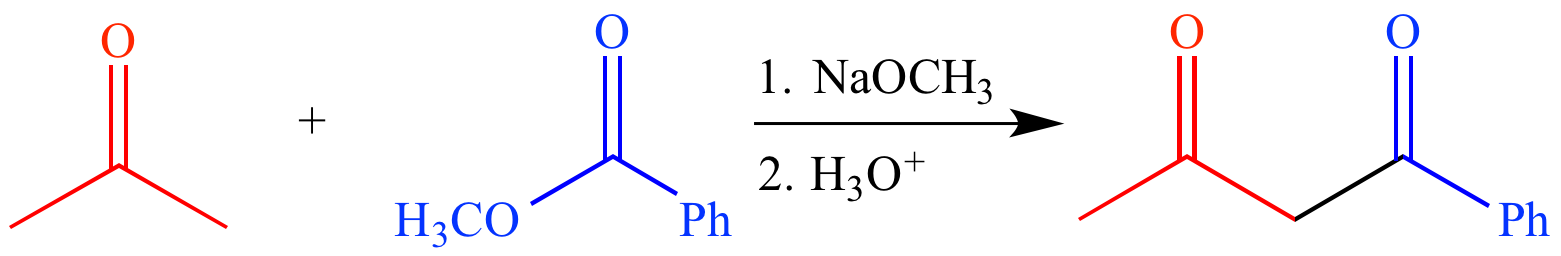
In a mixed Claisen condensation (or crossed Claisen condensation), an ester enolate or ketone enolate is condensed with an ester that cannot form an enolate. For example, reaction of acetone (a ketone which can form an enolate) and methyl benzoate (an ester which cannot form an enolate) with sodium methoxide (a strong base) followed by aqueous acid forms 1-phenylbutane-1,3-dione, a β-diketone.
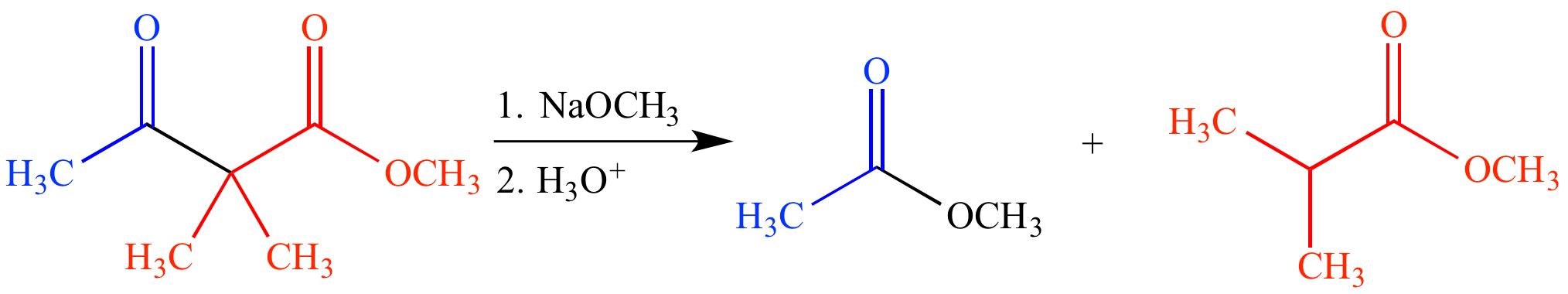
As the name implies, a retro-Claisen condensation is the reverse of a Claisen condensation. In this reaction a β-keto ester (or its enol tautomer) is reacted with an excess of strong base, causing fragmentation, and producing two ester products. The β-keto ester must be nonenolizable (lack a proton on the carbon between the two carbonyl groups), otherwise an enolate is formed and no fragmentation occurs.