| Photolysis
of molecular
bromine is a chain
initiation step. There are no radical
reactants,
and two radical
products. |
|
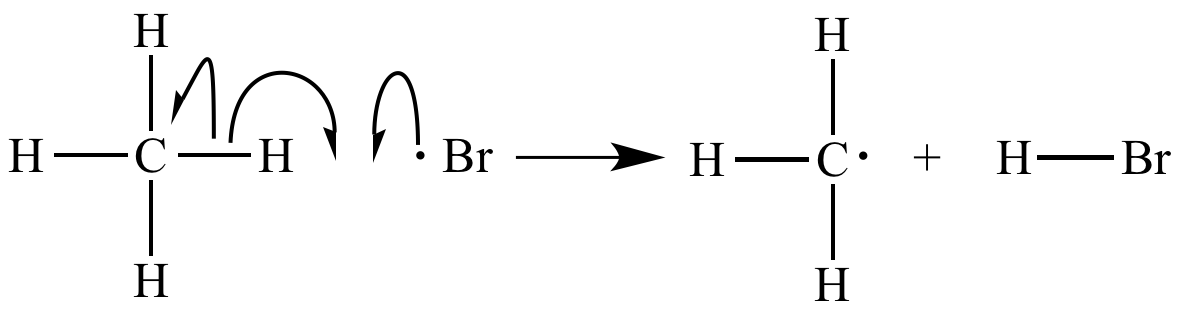 |
Hydrogen
atom
abstraction by bromine radical
is a chain
propagation step. |
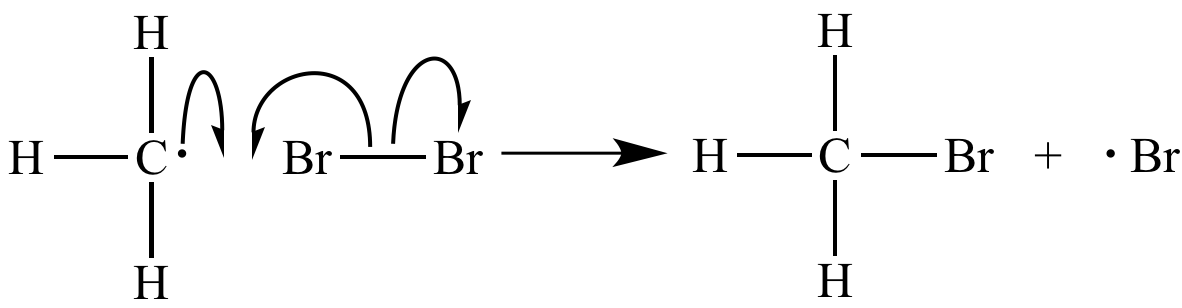 |
Bromine
atom
abstraction is a chain
propagation step. |
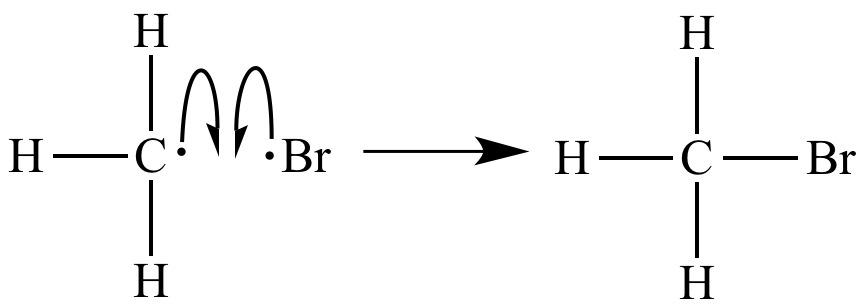 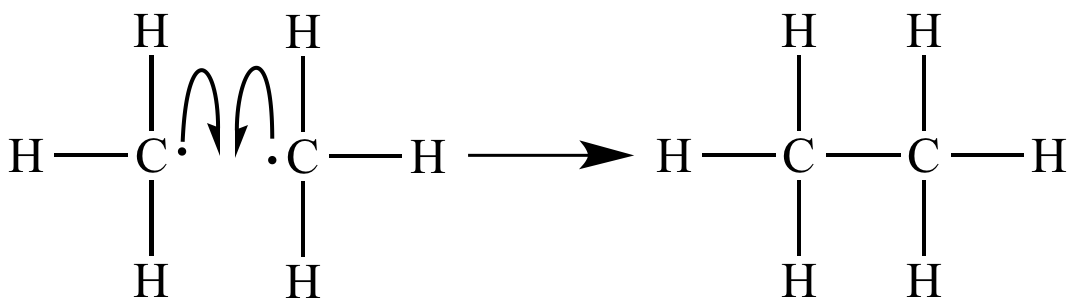 |
Several chain termination events are also occurring. |