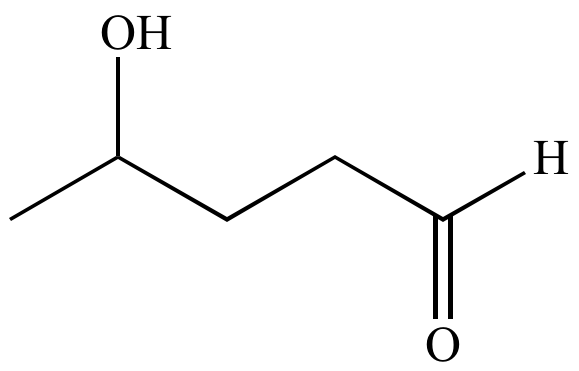
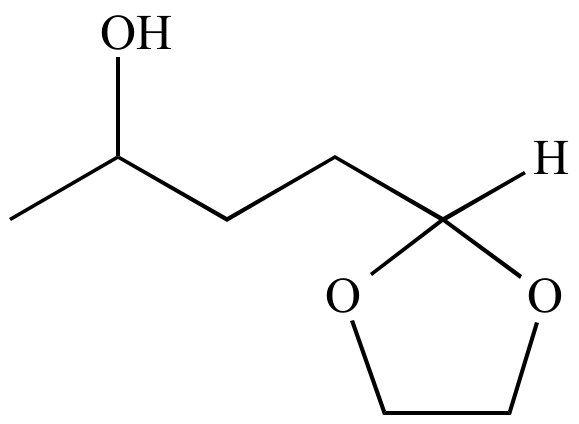
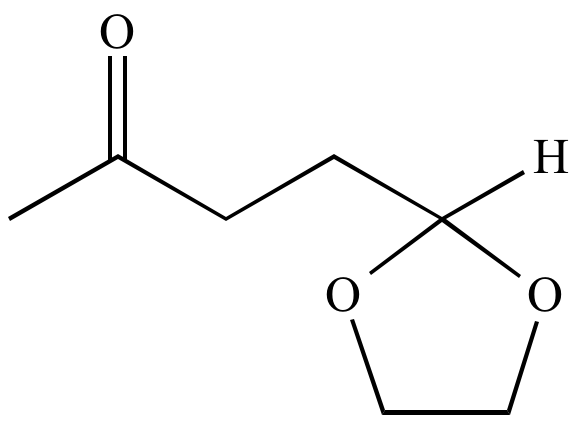
Atom economy:
A concept to measure the efficiency of a
synthetic
process, in terms of the number of atoms required in all the
starting
materials and
reactants
versus how many of these atoms are wasted (i.e., atoms that do not
become part of the final
product).
A process which uses many atoms not found in the final
product
is said to have low atom economy, whereas a process in which most
atoms of the
starting
material and
reactants
end up in the final
product
is said to have high atom economy. Processes having high atom
economy are generally (but not always) preferable to processes
having low atom economy.